Last Updated: December 8, 2022
Part 4: Clustering
Load libraries
library(Seurat)
library(ggplot2)
library(dplyr)
Load the Seurat object
load(file="pca_sample_corrected.RData")
experiment.aggregate
## An object of class Seurat
## 21005 features across 10595 samples within 1 assay
## Active assay: RNA (21005 features, 5986 variable features)
## 1 dimensional reduction calculated: pca
So how many features should we use? Use too few and your leaving out interesting variation that may define cell types, use too many and you add in noise? maybe?
Lets choose the first 25, based on our prior part.
use.pcs = 1:25
Identifying clusters
Seurat implements an graph-based clustering approach. Distances between the cells are calculated based on previously identified PCs.
The default method for identifying k-nearest neighbors has been changed in V4 to annoy (“Approximate Nearest Neighbors Oh Yeah!). This is an approximate nearest-neighbor approach that is widely used for high-dimensional analysis in many fields, including single-cell analysis. Extensive community benchmarking has shown that annoy substantially improves the speed and memory requirements of neighbor discovery, with negligible impact to downstream results.
Seurat prior approach was heavily inspired by recent manuscripts which applied graph-based clustering approaches to scRNAseq data. Briefly, Seurat identified clusters of cells by a shared nearest neighbor (SNN) modularity optimization based clustering algorithm. First calculate k-nearest neighbors (KNN) and construct the SNN graph. Then optimize the modularity function to determine clusters. For a full description of the algorithms, see Waltman and van Eck (2013) The European Physical Journal B. You can switch back to using the previous default setting using nn.method=”rann”.
The FindClusters function implements the neighbor based clustering procedure, and contains a resolution parameter that sets the granularity of the downstream clustering, with increased values leading to a greater number of clusters. I tend to like to perform a series of resolutions, investigate and choose.
?FindNeighbors
experiment.aggregate <- FindNeighbors(experiment.aggregate, reduction="pca", dims = use.pcs)
experiment.aggregate <- FindClusters(
object = experiment.aggregate,
resolution = seq(0.25,4,0.5),
verbose = FALSE
)
Seurat add the clustering information to the metadata beginning with RNA_snn_res. followed by the resolution
head(experiment.aggregate[[]])
## orig.ident nCount_RNA nFeature_RNA percent.mito
## AAACCCAAGTTATGGA_A001-C-007 A001-C-007 2076 1547 0.5780347
## AAACCCACAACGCCCA_A001-C-007 A001-C-007 854 687 1.5222482
## AAACCCACAGAAGTTA_A001-C-007 A001-C-007 540 466 1.6666667
## AAACCCACATGATAGA_A001-C-007 A001-C-007 514 438 3.5019455
## AAACCCAGTCAGTCCG_A001-C-007 A001-C-007 605 538 0.9917355
## AAACGAAGTTGGTGTT_A001-C-007 A001-C-007 948 766 0.0000000
## S.Score G2M.Score Phase old.ident
## AAACCCAAGTTATGGA_A001-C-007 0.022821930 -0.104890463 S A001-C-007
## AAACCCACAACGCCCA_A001-C-007 -0.008428992 0.123912762 G2M A001-C-007
## AAACCCACAGAAGTTA_A001-C-007 0.016243559 -0.055675102 S A001-C-007
## AAACCCACATGATAGA_A001-C-007 0.095646766 0.173522408 G2M A001-C-007
## AAACCCAGTCAGTCCG_A001-C-007 0.162755666 -0.009395696 S A001-C-007
## AAACGAAGTTGGTGTT_A001-C-007 -0.074341196 -0.047294231 G1 A001-C-007
## RNA_snn_res.0.25 RNA_snn_res.0.75 RNA_snn_res.1.25
## AAACCCAAGTTATGGA_A001-C-007 0 1 7
## AAACCCACAACGCCCA_A001-C-007 6 10 15
## AAACCCACAGAAGTTA_A001-C-007 9 13 12
## AAACCCACATGATAGA_A001-C-007 7 11 10
## AAACCCAGTCAGTCCG_A001-C-007 3 3 1
## AAACGAAGTTGGTGTT_A001-C-007 11 16 17
## RNA_snn_res.1.75 RNA_snn_res.2.25 RNA_snn_res.2.75
## AAACCCAAGTTATGGA_A001-C-007 10 15 17
## AAACCCACAACGCCCA_A001-C-007 18 17 23
## AAACCCACAGAAGTTA_A001-C-007 13 12 13
## AAACCCACATGATAGA_A001-C-007 9 9 8
## AAACCCAGTCAGTCCG_A001-C-007 1 1 4
## AAACGAAGTTGGTGTT_A001-C-007 21 21 22
## RNA_snn_res.3.25 RNA_snn_res.3.75 seurat_clusters
## AAACCCAAGTTATGGA_A001-C-007 17 20 20
## AAACCCACAACGCCCA_A001-C-007 23 24 24
## AAACCCACAGAAGTTA_A001-C-007 12 12 12
## AAACCCACATGATAGA_A001-C-007 19 22 22
## AAACCCAGTCAGTCCG_A001-C-007 4 7 7
## AAACGAAGTTGGTGTT_A001-C-007 22 23 23
Lets first investigate how many clusters each resolution produces and set it to the smallest resolutions of 0.5 (fewest clusters).
sapply(grep("res",colnames(experiment.aggregate@meta.data),value = TRUE),
function(x) length(unique(experiment.aggregate@meta.data[,x])))
## RNA_snn_res.0.25 RNA_snn_res.0.75 RNA_snn_res.1.25 RNA_snn_res.1.75
## 17 24 27 33
## RNA_snn_res.2.25 RNA_snn_res.2.75 RNA_snn_res.3.25 RNA_snn_res.3.75
## 33 37 40 43
Plot TSNE coloring for each resolution
tSNE dimensionality reduction plots are then used to visualize clustering results. As input to the tSNE, you should use the same PCs as input to the clustering analysis.
experiment.aggregate <- RunTSNE(
object = experiment.aggregate,
reduction.use = "pca",
dims = use.pcs,
do.fast = TRUE)
DimPlot(object = experiment.aggregate, group.by=grep("res",colnames(experiment.aggregate@meta.data),value = TRUE)[1:4], ncol=2 , pt.size=3.0, reduction = "tsne", label = T)
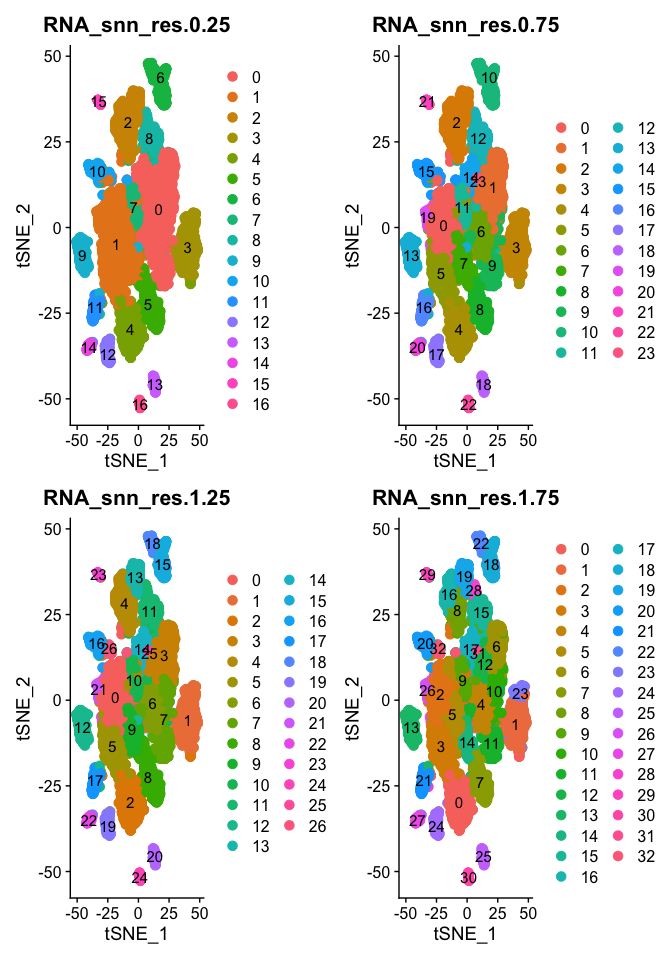
DimPlot(object = experiment.aggregate, group.by=grep("res",colnames(experiment.aggregate@meta.data),value = TRUE)[5:8], ncol=2 , pt.size=3.0, reduction = "tsne", label = T)
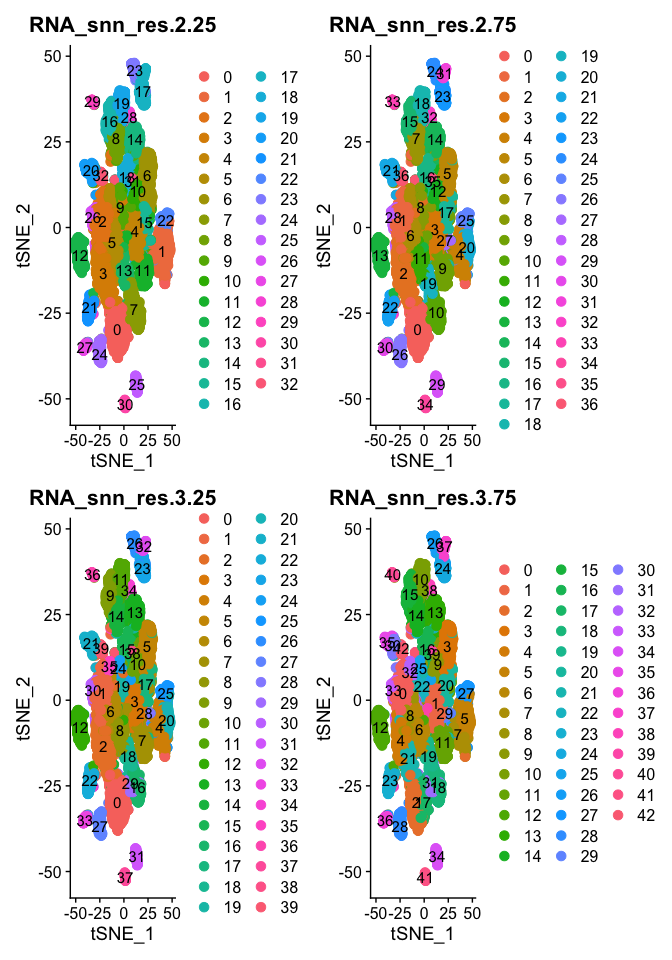
- Try exploring different PCS, so first 5, 10, 15, we used 25, what about 100? How does the clustering change?
Once complete go back to 1:25
Choosing a resolution
Lets set the default identity to a resolution of 1.25 and produce a table of cluster to sample assignments.
Idents(experiment.aggregate) <- "RNA_snn_res.1.25"
table(Idents(experiment.aggregate),experiment.aggregate$orig.ident)
##
## A001-C-007 A001-C-104 B001-A-301
## 0 0 2 1102
## 1 922 1 0
## 2 3 1 891
## 3 89 787 4
## 4 3 3 684
## 5 1 4 677
## 6 13 660 1
## 7 234 402 4
## 8 1 16 617
## 9 0 1 404
## 10 14 380 7
## 11 56 331 2
## 12 83 164 83
## 13 2 1 269
## 14 45 226 0
## 15 114 75 81
## 16 1 5 203
## 17 96 66 34
## 18 2 57 98
## 19 76 43 27
## 20 6 46 40
## 21 1 2 80
## 22 4 48 28
## 23 2 46 1
## 24 4 9 31
## 25 2 39 0
## 26 0 1 37
Plot TSNE coloring by the slot ‘ident’ (default).
DimPlot(object = experiment.aggregate, pt.size=0.5, reduction = "tsne", label = T)
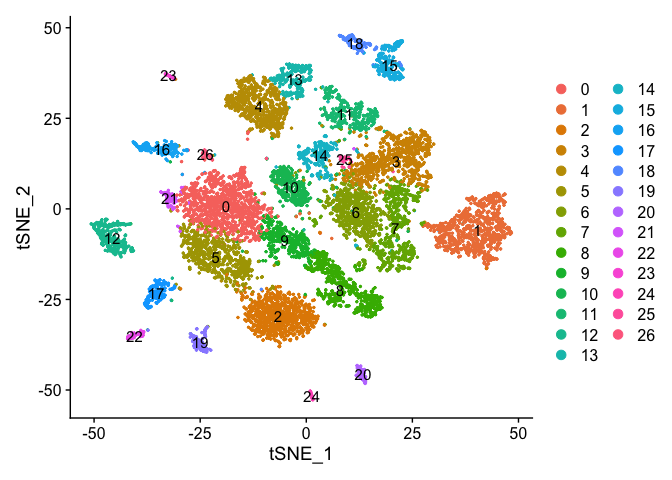
UMAP dimensionality reduction plot.
experiment.aggregate <- RunUMAP(
object = experiment.aggregate,
dims = use.pcs)
Plot UMAP coloring by the slot ‘ident’ (default).
DimPlot(object = experiment.aggregate, pt.size=0.5, reduction = "umap", label = T)
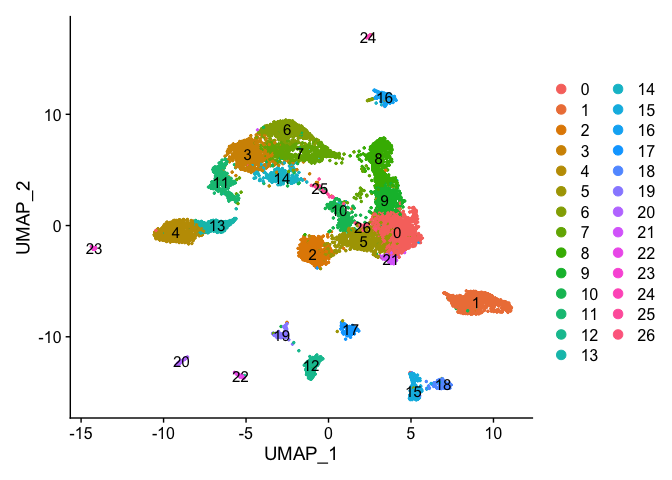
Catagorical data can be plotted using the DimPlot function.
TSNE plot by cell cycle
DimPlot(object = experiment.aggregate, pt.size=0.5, group.by = "Phase", reduction = "umap")
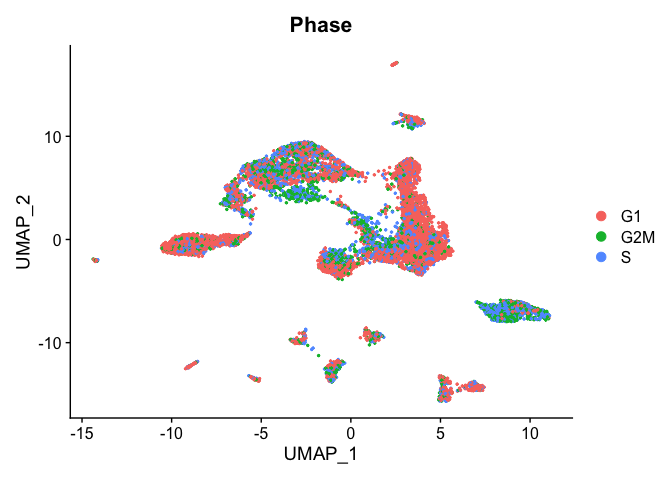
- Try creating a table, of cluster x cell cycle
Can use feature plot to plot our read valued metadata, like nUMI, Feature count, and percent Mito
FeaturePlot can be used to color cells with a ‘feature’, non categorical data, like number of UMIs
FeaturePlot(experiment.aggregate, features = c('nCount_RNA'), pt.size=0.5)
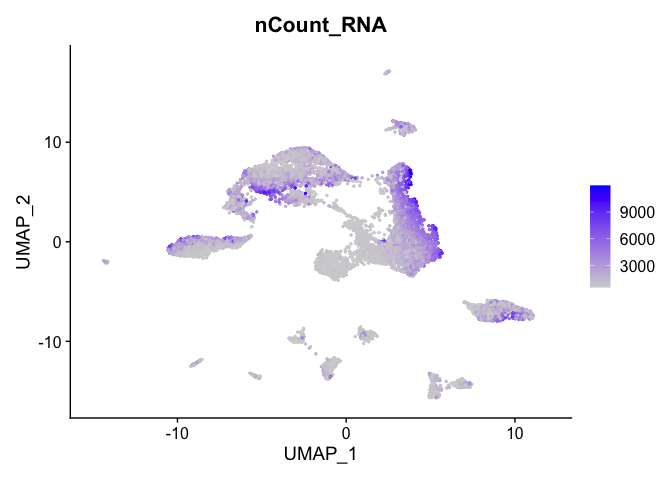
and number of genes present
FeaturePlot(experiment.aggregate, features = c('nFeature_RNA'), pt.size=0.5)
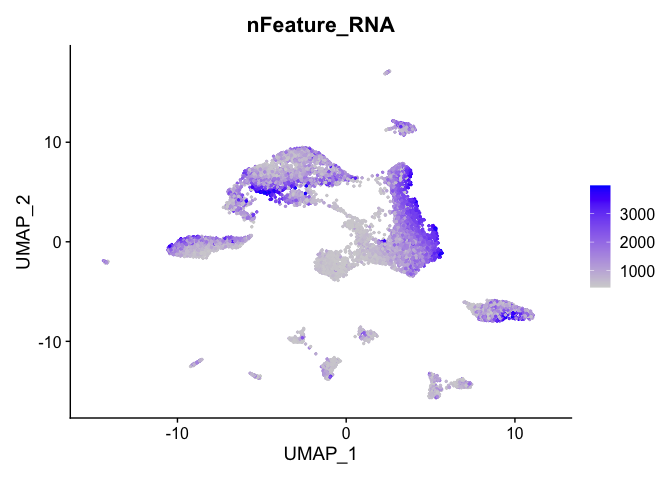
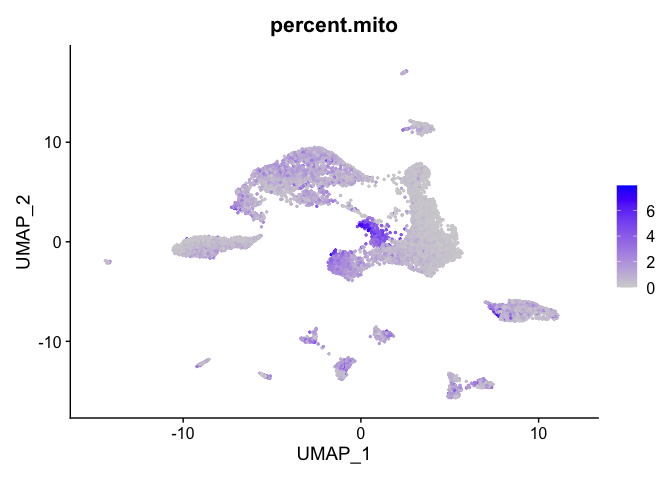
percent mitochondrial
FeaturePlot(experiment.aggregate, features = c('percent.mito'), pt.size=0.5)
Building a phylogenetic tree relating the ‘average’ cell from each group in default ‘Ident’ (currently “RNA_snn_res.1.25”). Tree is estimated based on a distance matrix constructed in either gene expression space or PCA space.
Idents(experiment.aggregate) <- "RNA_snn_res.1.25"
experiment.aggregate <- BuildClusterTree(
experiment.aggregate, dims = use.pcs)
PlotClusterTree(experiment.aggregate)
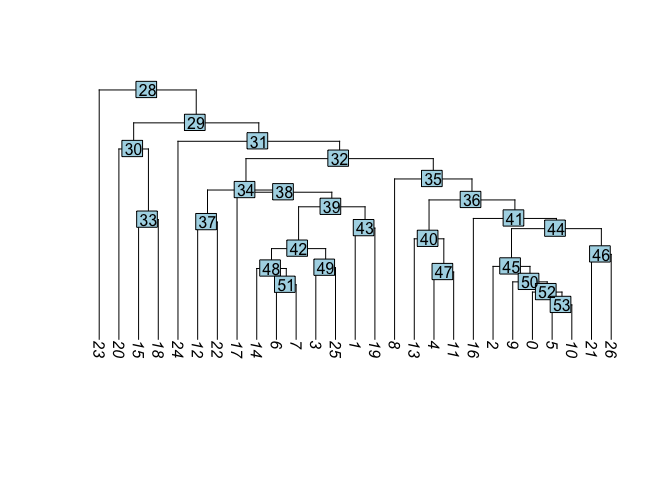
- Create new trees of other data
Once complete go back to Res 1.25
DimPlot(object = experiment.aggregate, pt.size=0.5, label = TRUE, reduction = "umap")
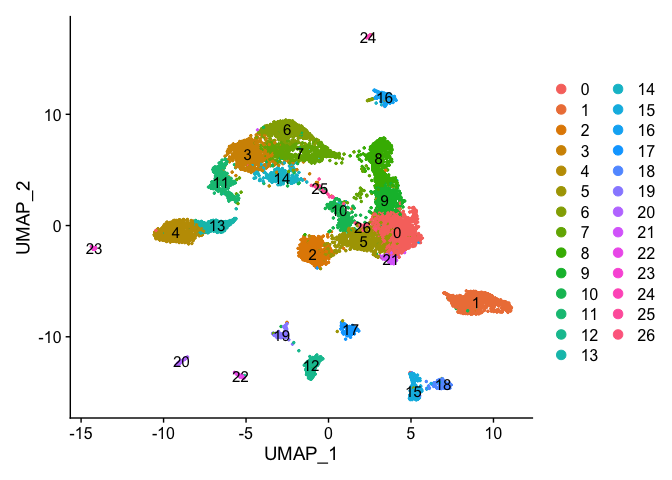
DimPlot(experiment.aggregate, pt.size = 0.5, label = TRUE, reduction = "tsne")
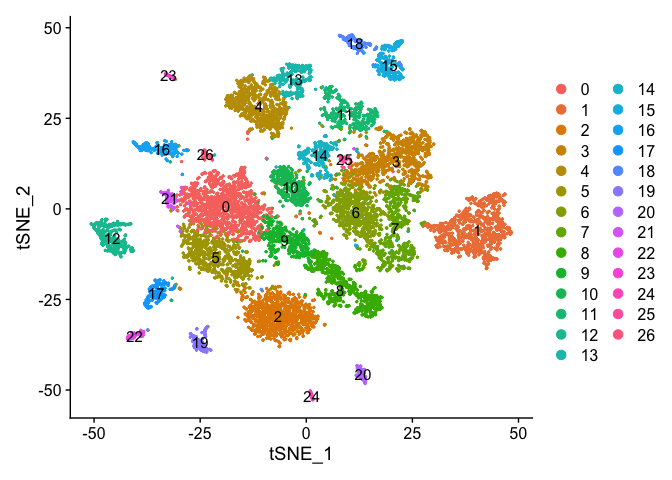
Merging clusters
Merge Clustering results, so lets say clusters 0, and 21 are actually the same cell type and we don’t wish to separate them out as distinct clusters. Same with 3, and 25.
experiment.merged = experiment.aggregate
Idents(experiment.merged) <- "RNA_snn_res.1.25"
experiment.merged <- RenameIdents(
object = experiment.merged,
'21' = '0',
'25' = '3'
)
table(Idents(experiment.merged))
##
## 0 3 1 2 4 5 6 7 8 9 10 11 12 13 14 15
## 1187 921 923 895 690 682 674 640 634 405 401 389 330 272 271 270
## 16 17 18 19 20 22 23 24 26
## 209 196 157 146 92 80 49 44 38
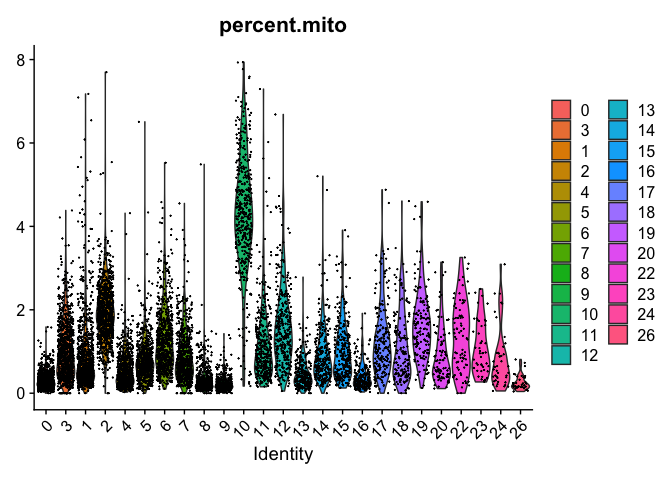
DimPlot(object = experiment.merged, pt.size=0.5, label = T, reduction = "umap")
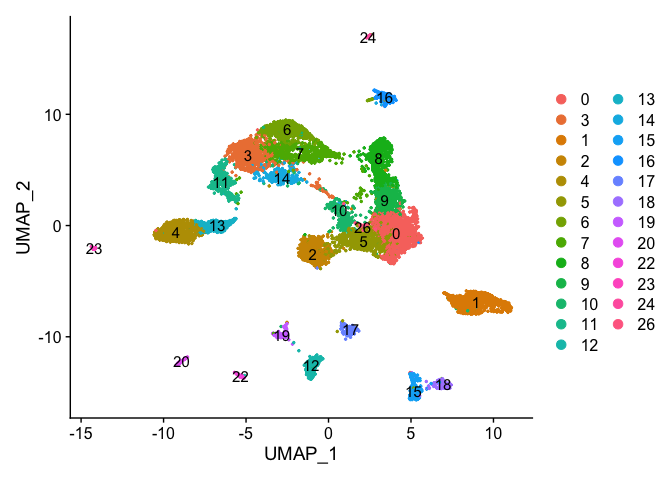
DimPlot(experiment.merged, pt.size = 0.5, label = TRUE, reduction = "tsne" )
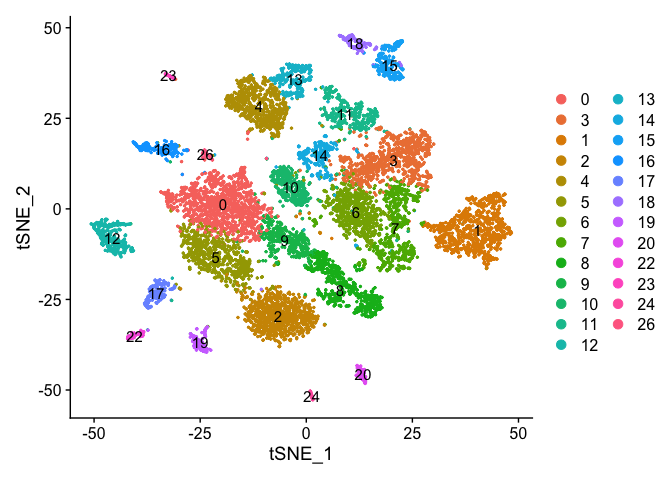
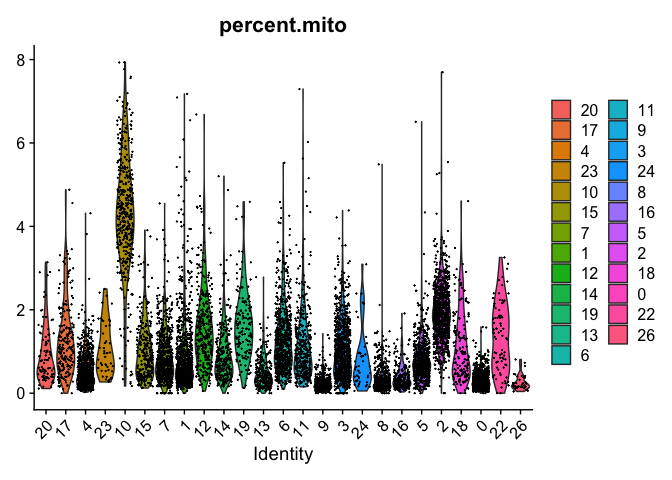
VlnPlot(object = experiment.merged, features = "percent.mito", pt.size = 0.05)
Reordering the clusters
In order to reorder the clusters for plotting purposes take a look at the levels of the Ident, which indicates the ordering, then relevel as desired.
experiment.examples <- experiment.merged
levels(experiment.examples@active.ident)
## [1] "0" "3" "1" "2" "4" "5" "6" "7" "8" "9" "10" "11" "12" "13" "14"
## [16] "15" "16" "17" "18" "19" "20" "22" "23" "24" "26"
experiment.examples@active.ident <- relevel(experiment.examples@active.ident, "12")
levels(experiment.examples@active.ident)
## [1] "12" "0" "3" "1" "2" "4" "5" "6" "7" "8" "9" "10" "11" "13" "14"
## [16] "15" "16" "17" "18" "19" "20" "22" "23" "24" "26"
# now cluster 12 is the "first" factor
DimPlot(object = experiment.examples, pt.size=0.5, label = T, reduction = "umap")
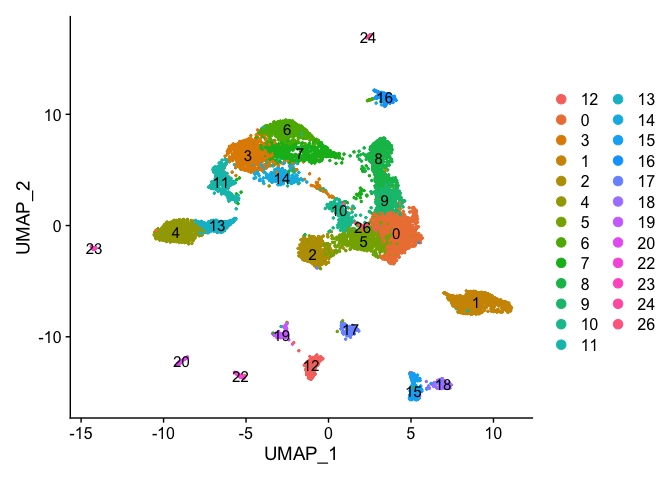
VlnPlot(object = experiment.examples, features = "percent.mito", pt.size = 0.05)
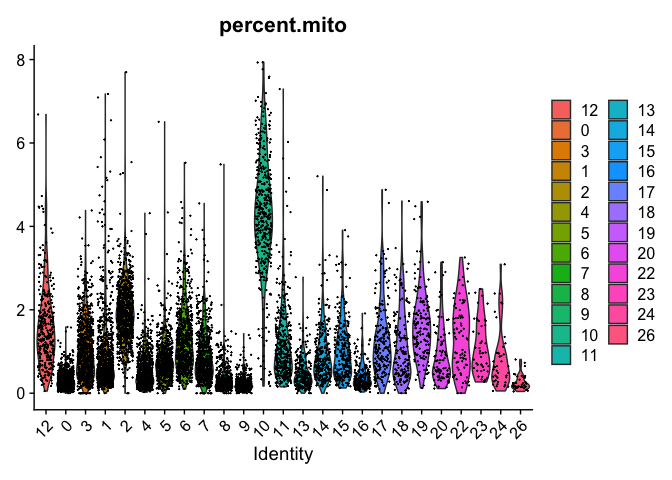
# relevel all the factors to the order I want
neworder <- sample(levels(experiment.examples), replace=FALSE)
Idents(experiment.examples) <- factor(experiment.examples@active.ident, levels=neworder)
levels(experiment.examples@active.ident)
## [1] "20" "17" "4" "23" "10" "15" "7" "1" "12" "14" "19" "13" "6" "11" "9"
## [16] "3" "24" "8" "16" "5" "2" "18" "0" "22" "26"
DimPlot(object = experiment.examples, pt.size=0.5, label = T, reduction = "umap")
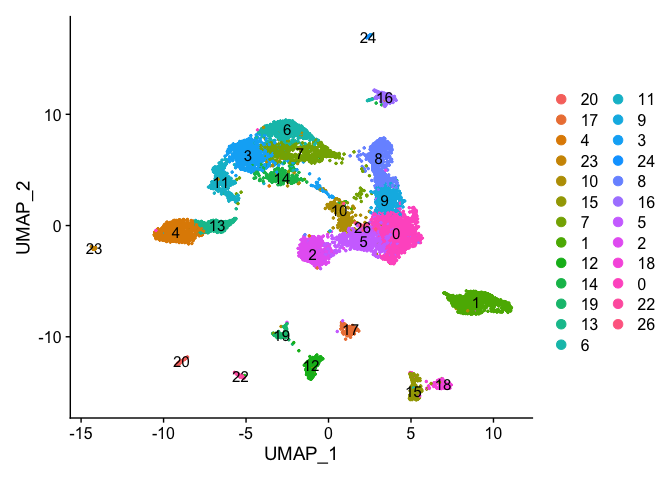
VlnPlot(object = experiment.examples, features = "percent.mito", pt.size = 0.05)
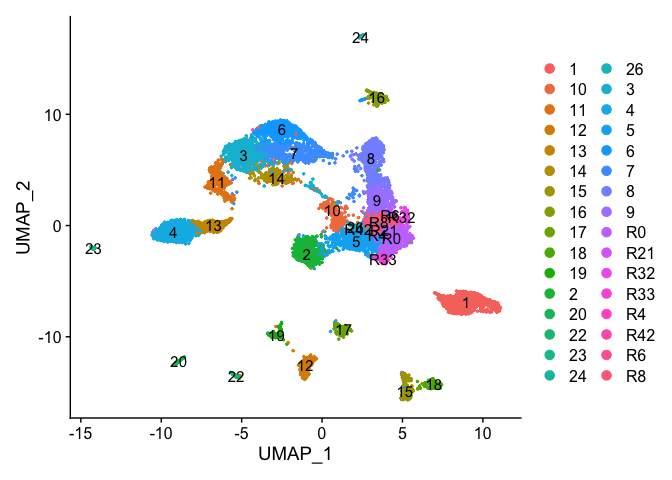
Re-assign clustering result (subclustering only cluster 0) to clustering for resolution 3.75 (@ reslution 0.25) [adding a R prefix]
newIdent = as.character(Idents(experiment.examples))
newIdent[newIdent == '0'] = paste0("R",as.character(experiment.examples$RNA_snn_res.3.75[newIdent == '0']))
Idents(experiment.examples) <- as.factor(newIdent)
table(Idents(experiment.examples))
##
## 1 10 11 12 13 14 15 16 17 18 19 2 20 22 23 24 26 3 4 5
## 923 401 389 330 272 271 270 209 196 157 146 895 92 80 49 44 38 921 690 682
## 6 7 8 9 R0 R21 R32 R33 R4 R42 R6 R8
## 674 640 634 405 668 4 105 91 5 1 2 311
DimPlot(object = experiment.examples, pt.size=0.5, label = T, reduction = "umap")
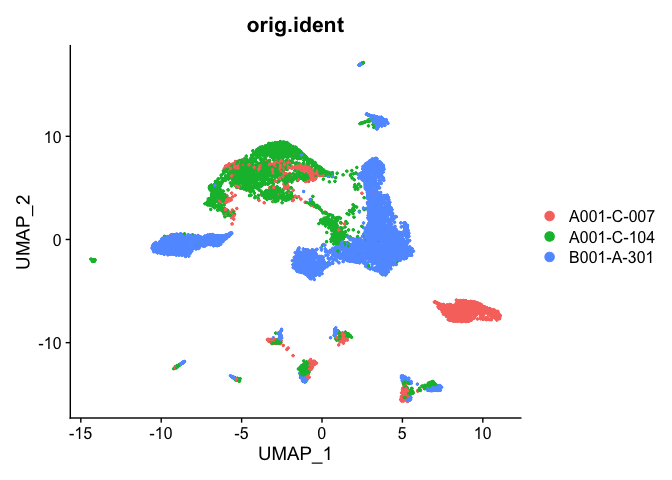
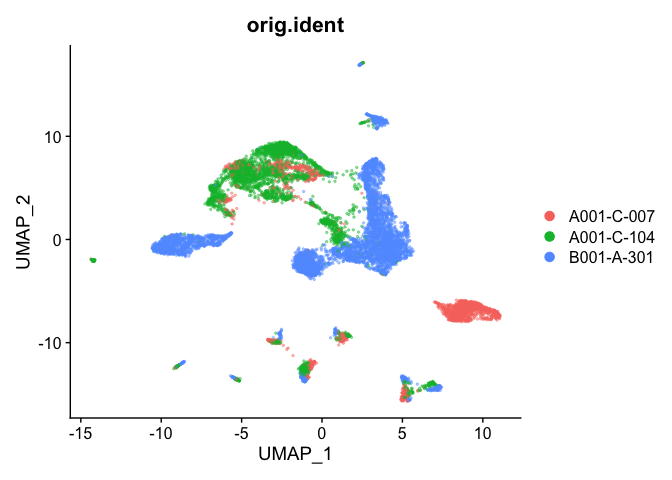
Plot UMAP coloring by the slot ‘orig.ident’ (sample names) with alpha colors turned on. A pretty picture
DimPlot(object = experiment.aggregate, group.by="orig.ident", pt.size=0.5, reduction = "umap", shuffle = TRUE)
## Pretty umap using alpha
alpha.use <- 2/5
p <- DimPlot(object = experiment.aggregate, group.by="orig.ident", pt.size=0.5, reduction = "umap", shuffle = TRUE)
p$layers[[1]]$mapping$alpha <- alpha.use
p + scale_alpha_continuous(range = alpha.use, guide = F)
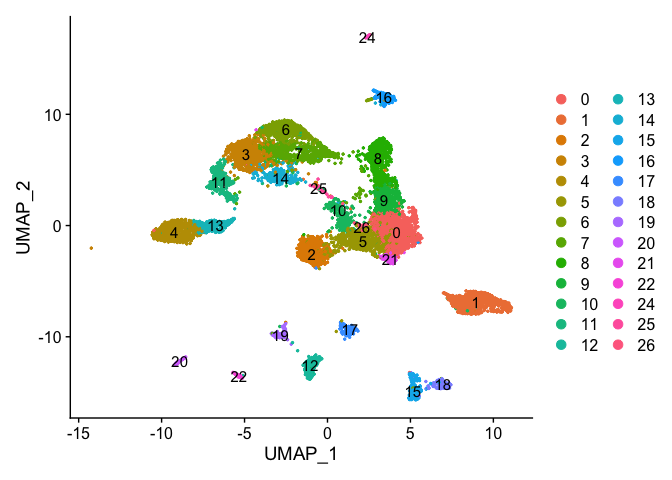
Removing cells assigned to clusters from a plot, So here plot all clusters but cluster 23 (contaminant?)
# create a new tmp object with those removed
experiment.aggregate.tmp <- experiment.aggregate[,-which(Idents(experiment.aggregate) %in% c("23"))]
dim(experiment.aggregate)
## [1] 21005 10595
dim(experiment.aggregate.tmp)
## [1] 21005 10546
DimPlot(object = experiment.aggregate.tmp, pt.size=0.5, reduction = "umap", label = T)
Identifying Marker Genes
Seurat can help you find markers that define clusters via differential expression.
FindMarkers identifies markers for a cluster relative to all other clusters.
FindAllMarkers does so for all clusters
FindAllMarkersNode defines all markers that split a Node from the cluster tree
?FindMarkers
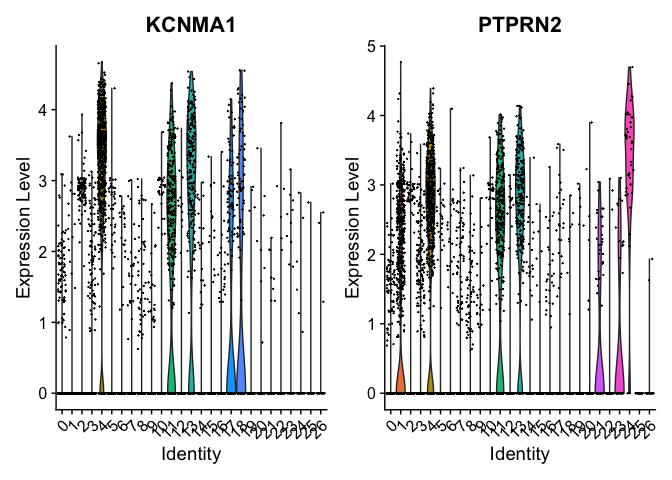
markers = FindMarkers(experiment.aggregate, ident.1=c(4,13), ident.2 = c(6,7))
head(markers)
## p_val avg_log2FC pct.1 pct.2 p_val_adj
## KCNMA1 0.000000e+00 4.521474 0.856 0.034 0.000000e+00
## PTPRN2 5.411145e-293 3.441014 0.812 0.063 1.136611e-288
## FCGBP 8.092151e-287 3.416703 0.859 0.133 1.699756e-282
## NEDD4L 1.124816e-279 2.858062 0.941 0.460 2.362675e-275
## MUC2 4.305183e-253 2.915768 0.840 0.141 9.043036e-249
## XIST 5.010454e-243 3.027981 0.682 0.018 1.052446e-238
dim(markers)
## [1] 2270 5
table(markers$avg_log2FC > 0)
##
## FALSE TRUE
## 1229 1041
table(markers$p_val_adj < 0.05 & markers$avg_log2FC > 0)
##
## FALSE TRUE
## 1423 847
pct.1 and pct.2 are the proportion of cells with expression above 0 in ident.1 and ident.2 respectively. p_val is the raw p_value associated with the differntial expression test with adjusted value in p_val_adj. avg_logFC is the average log fold change difference between the two groups.
Can use a violin plot to visualize the expression pattern of some markers
VlnPlot(object = experiment.aggregate, features = rownames(markers)[1:2], pt.size = 0.05)
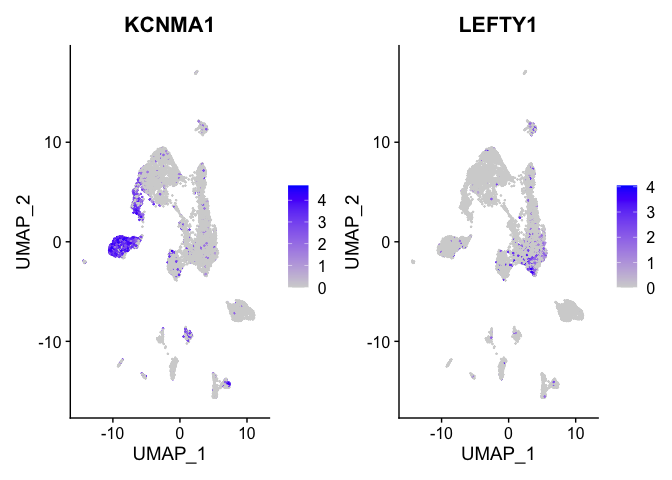
Or a feature plot
FeaturePlot(
experiment.aggregate,
features = c("KCNMA1", "LEFTY1"),
cols = c("lightgrey", "blue"),
ncol = 2
)
FindAllMarkers can be used to automate the process across all genes.
markers_all <- FindAllMarkers(
object = experiment.merged,
only.pos = TRUE,
min.pct = 0.25,
thresh.use = 0.25
)
dim(markers_all)
## [1] 10418 7
head(markers_all)
## p_val avg_log2FC pct.1 pct.2 p_val_adj cluster gene
## RBFOX1 0 1.801003 0.891 0.315 0 0 RBFOX1
## NXPE1 0 1.609535 0.970 0.495 0 0 NXPE1
## ADAMTSL1 0 1.599482 0.935 0.410 0 0 ADAMTSL1
## DPP10 0 1.434679 0.643 0.154 0 0 DPP10
## EYA2 0 1.422714 0.496 0.085 0 0 EYA2
## XIST 0 1.396557 0.896 0.281 0 0 XIST
table(table(markers_all$gene))
##
## 1 2 3 4 5 6 7 8 9 10 11 12
## 1453 863 680 460 302 170 76 24 8 1 1 1
markers_all_single <- markers_all[markers_all$gene %in% names(table(markers_all$gene))[table(markers_all$gene) == 1],]
dim(markers_all_single)
## [1] 1453 7
table(table(markers_all_single$gene))
##
## 1
## 1453
table(markers_all_single$cluster)
##
## 0 3 1 2 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19
## 27 32 177 15 22 0 4 20 197 82 3 12 23 44 46 28 72 42 41 23
## 20 22 23 24 26
## 81 31 87 71 273
head(markers_all_single)
## p_val avg_log2FC pct.1 pct.2 p_val_adj cluster gene
## LEFTY1 1.382475e-158 0.8183984 0.256 0.044 2.903889e-154 0 LEFTY1
## ATP13A4 8.738362e-133 0.6535469 0.264 0.056 1.835493e-128 0 ATP13A4
## MYB 1.904969e-124 0.6899936 0.386 0.117 4.001387e-120 0 MYB
## NDUFAF2 1.026241e-81 0.5774651 0.302 0.101 2.155619e-77 0 NDUFAF2
## AKAP7 1.830143e-68 0.4202497 0.317 0.119 3.844215e-64 0 AKAP7
## LRATD1 7.161102e-56 0.3635457 0.288 0.115 1.504189e-51 0 LRATD1
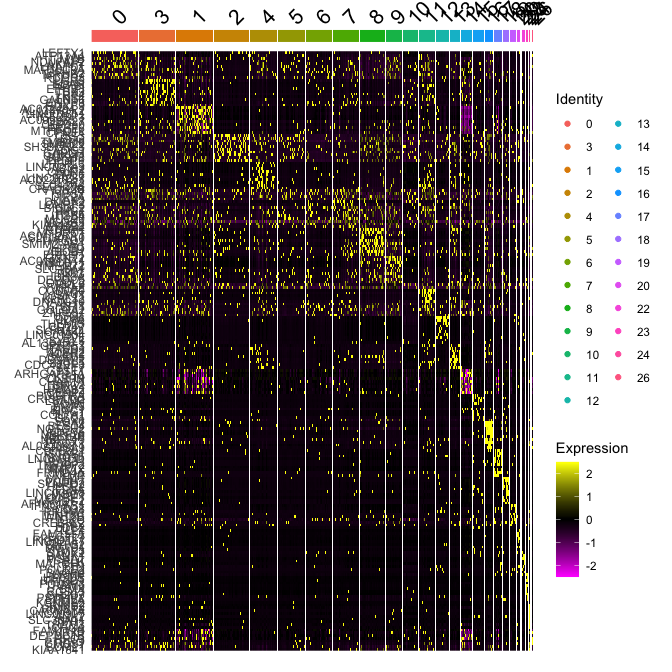
Plot a heatmap of genes by cluster for the top 10 marker genes per cluster
top10 <- markers_all_single %>% group_by(cluster) %>% top_n(10, avg_log2FC)
DoHeatmap(
object = experiment.merged,
features = top10$gene
)
# Get expression of genes for cells in and out of each cluster
getGeneClusterMeans <- function(gene, cluster){
x <- GetAssayData(experiment.merged)[gene,]
m <- tapply(x, ifelse(Idents(experiment.merged) == cluster, 1, 0), mean)
mean.in.cluster <- m[2]
mean.out.of.cluster <- m[1]
return(list(mean.in.cluster = mean.in.cluster, mean.out.of.cluster = mean.out.of.cluster))
}
## for sake of time only using first six (head)
means <- mapply(getGeneClusterMeans, head(markers_all[,"gene"]), head(markers_all[,"cluster"]))
means <- matrix(unlist(means), ncol = 2, byrow = T)
colnames(means) <- c("mean.in.cluster", "mean.out.of.cluster")
rownames(means) <- head(markers_all[,"gene"])
markers_all2 <- cbind(head(markers_all), means)
head(markers_all2)
## p_val avg_log2FC pct.1 pct.2 p_val_adj cluster gene
## RBFOX1 0 1.801003 0.891 0.315 0 0 RBFOX1
## NXPE1 0 1.609535 0.970 0.495 0 0 NXPE1
## ADAMTSL1 0 1.599482 0.935 0.410 0 0 ADAMTSL1
## DPP10 0 1.434679 0.643 0.154 0 0 DPP10
## EYA2 0 1.422714 0.496 0.085 0 0 EYA2
## XIST 0 1.396557 0.896 0.281 0 0 XIST
## mean.in.cluster mean.out.of.cluster
## RBFOX1 2.5396188 0.7812035
## NXPE1 3.1683411 1.3435546
## ADAMTSL1 2.8933510 1.0967794
## DPP10 1.3751032 0.3315594
## EYA2 0.9699287 0.1683474
## XIST 2.3334639 0.7333243
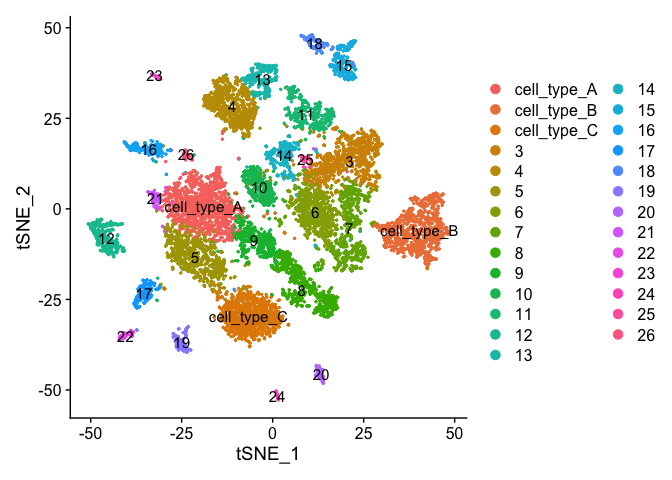
Finishing up clusters.
At this point in time you should use the tree, markers, domain knowledge, and goals to finalize your clusters. This may mean adjusting PCA to use, mergers clusters together, choosing a new resolutions, etc. When finished you can further name it cluster by something more informative. Ex.
experiment.clusters <- experiment.aggregate
experiment.clusters <- RenameIdents(
object = experiment.clusters,
'0' = 'cell_type_A',
'1' = 'cell_type_B',
'2' = 'cell_type_C'
)
# and so on
DimPlot(object = experiment.clusters, pt.size=0.5, label = T, reduction = "tsne")
Right now our results ONLY exist in the Ident data object, lets save it to our metadata table so we don’t accidentally loose it.
experiment.merged$finalcluster <- Idents(experiment.merged)
head(experiment.merged[[]])
## orig.ident nCount_RNA nFeature_RNA percent.mito
## AAACCCAAGTTATGGA_A001-C-007 A001-C-007 2076 1547 0.5780347
## AAACCCACAACGCCCA_A001-C-007 A001-C-007 854 687 1.5222482
## AAACCCACAGAAGTTA_A001-C-007 A001-C-007 540 466 1.6666667
## AAACCCACATGATAGA_A001-C-007 A001-C-007 514 438 3.5019455
## AAACCCAGTCAGTCCG_A001-C-007 A001-C-007 605 538 0.9917355
## AAACGAAGTTGGTGTT_A001-C-007 A001-C-007 948 766 0.0000000
## S.Score G2M.Score Phase old.ident
## AAACCCAAGTTATGGA_A001-C-007 0.022821930 -0.104890463 S A001-C-007
## AAACCCACAACGCCCA_A001-C-007 -0.008428992 0.123912762 G2M A001-C-007
## AAACCCACAGAAGTTA_A001-C-007 0.016243559 -0.055675102 S A001-C-007
## AAACCCACATGATAGA_A001-C-007 0.095646766 0.173522408 G2M A001-C-007
## AAACCCAGTCAGTCCG_A001-C-007 0.162755666 -0.009395696 S A001-C-007
## AAACGAAGTTGGTGTT_A001-C-007 -0.074341196 -0.047294231 G1 A001-C-007
## RNA_snn_res.0.25 RNA_snn_res.0.75 RNA_snn_res.1.25
## AAACCCAAGTTATGGA_A001-C-007 0 1 7
## AAACCCACAACGCCCA_A001-C-007 6 10 15
## AAACCCACAGAAGTTA_A001-C-007 9 13 12
## AAACCCACATGATAGA_A001-C-007 7 11 10
## AAACCCAGTCAGTCCG_A001-C-007 3 3 1
## AAACGAAGTTGGTGTT_A001-C-007 11 16 17
## RNA_snn_res.1.75 RNA_snn_res.2.25 RNA_snn_res.2.75
## AAACCCAAGTTATGGA_A001-C-007 10 15 17
## AAACCCACAACGCCCA_A001-C-007 18 17 23
## AAACCCACAGAAGTTA_A001-C-007 13 12 13
## AAACCCACATGATAGA_A001-C-007 9 9 8
## AAACCCAGTCAGTCCG_A001-C-007 1 1 4
## AAACGAAGTTGGTGTT_A001-C-007 21 21 22
## RNA_snn_res.3.25 RNA_snn_res.3.75 seurat_clusters
## AAACCCAAGTTATGGA_A001-C-007 17 20 20
## AAACCCACAACGCCCA_A001-C-007 23 24 24
## AAACCCACAGAAGTTA_A001-C-007 12 12 12
## AAACCCACATGATAGA_A001-C-007 19 22 22
## AAACCCAGTCAGTCCG_A001-C-007 4 7 7
## AAACGAAGTTGGTGTT_A001-C-007 22 23 23
## finalcluster
## AAACCCAAGTTATGGA_A001-C-007 7
## AAACCCACAACGCCCA_A001-C-007 15
## AAACCCACAGAAGTTA_A001-C-007 12
## AAACCCACATGATAGA_A001-C-007 10
## AAACCCAGTCAGTCCG_A001-C-007 1
## AAACGAAGTTGGTGTT_A001-C-007 17
table(experiment.merged$finalcluster, experiment.merged$orig.ident)
##
## A001-C-007 A001-C-104 B001-A-301
## 0 1 4 1182
## 3 91 826 4
## 1 922 1 0
## 2 3 1 891
## 4 3 3 684
## 5 1 4 677
## 6 13 660 1
## 7 234 402 4
## 8 1 16 617
## 9 0 1 404
## 10 14 380 7
## 11 56 331 2
## 12 83 164 83
## 13 2 1 269
## 14 45 226 0
## 15 114 75 81
## 16 1 5 203
## 17 96 66 34
## 18 2 57 98
## 19 76 43 27
## 20 6 46 40
## 22 4 48 28
## 23 2 46 1
## 24 4 9 31
## 26 0 1 37
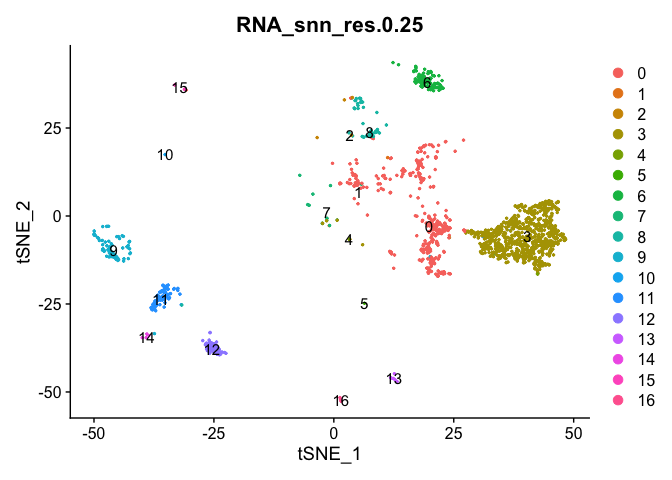
Subsetting samples and plotting
If you want to look at the representation of just one sample, or sets of samples
experiment.sample1 <- subset(experiment.merged, orig.ident == "A001-C-007")
DimPlot(object = experiment.sample1, group.by = "RNA_snn_res.0.25", pt.size=0.5, label = TRUE, reduction = "tsne")
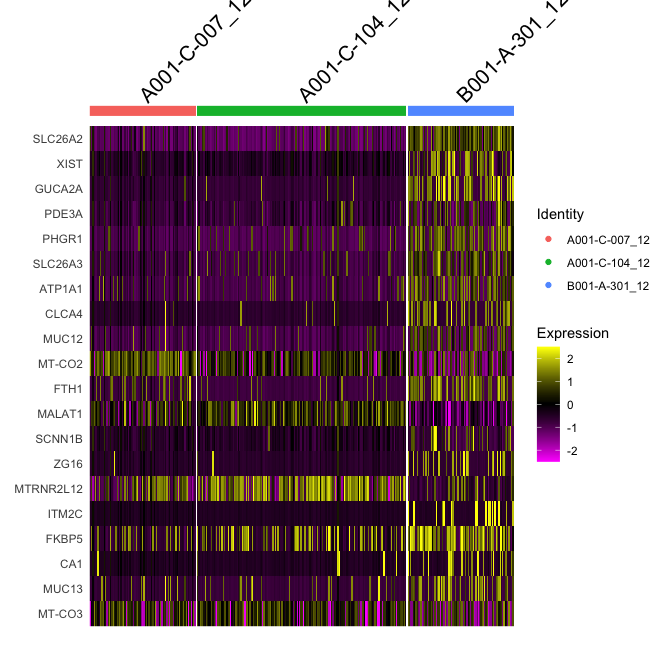
Adding in a new metadata column representing samples within clusters. So differential expression of A001-C-007 vs B001-A-301 within cluster 0
experiment.merged$samplecluster = paste(experiment.merged$orig.ident,experiment.merged$finalcluster,sep = '_')
# set the identity to the new variable
Idents(experiment.merged) <- "samplecluster"
markers.comp <- FindMarkers(experiment.merged, ident.1 = c("A001-C-007_12", "A001-C-104_12"), ident.2= "B001-A-301_12")
head(markers.comp)
## p_val avg_log2FC pct.1 pct.2 p_val_adj
## SLC26A2 1.103276e-42 -3.406530 0.138 0.904 2.317432e-38
## XIST 2.145342e-34 -3.351456 0.000 0.530 4.506291e-30
## GUCA2A 4.025474e-31 -3.190970 0.020 0.554 8.455508e-27
## PDE3A 8.506056e-27 -2.909730 0.032 0.530 1.786697e-22
## PHGR1 2.436331e-26 -2.546360 0.113 0.699 5.117514e-22
## SLC26A3 4.059153e-26 -2.597649 0.089 0.663 8.526252e-22
experiment.subset <- subset(experiment.merged, samplecluster %in% c( "A001-C-007_12", "A001-C-104_12", "B001-A-301_12" ))
DoHeatmap(object = experiment.subset, features = head(rownames(markers.comp),20))
Idents(experiment.merged) <- "finalcluster"
And last lets save all the Seurat objects in our session.
save(list=grep('experiment', ls(), value = TRUE), file="clusters_seurat_object.RData")
Get the next Rmd file
download.file("https://raw.githubusercontent.com/ucdavis-bioinformatics-training/2022-December-Single-Cell-RNA-Seq-Analysis/main/data_analysis/scRNA_Workshop-PART5.Rmd", "scRNA_Workshop-PART5.Rmd")
Session Information
sessionInfo()
## R version 4.2.2 (2022-10-31)
## Platform: x86_64-apple-darwin17.0 (64-bit)
## Running under: macOS Catalina 10.15.7
##
## Matrix products: default
## BLAS: /Library/Frameworks/R.framework/Versions/4.2/Resources/lib/libRblas.0.dylib
## LAPACK: /Library/Frameworks/R.framework/Versions/4.2/Resources/lib/libRlapack.dylib
##
## locale:
## [1] en_US.UTF-8/en_US.UTF-8/en_US.UTF-8/C/en_US.UTF-8/en_US.UTF-8
##
## attached base packages:
## [1] stats graphics grDevices utils datasets methods base
##
## other attached packages:
## [1] dplyr_1.0.10 ggplot2_3.4.0 SeuratObject_4.1.3 Seurat_4.3.0
##
## loaded via a namespace (and not attached):
## [1] ggbeeswarm_0.6.0 Rtsne_0.16 colorspace_2.0-3
## [4] deldir_1.0-6 ellipsis_0.3.2 ggridges_0.5.4
## [7] rstudioapi_0.14 spatstat.data_3.0-0 farver_2.1.1
## [10] leiden_0.4.3 listenv_0.8.0 ggrepel_0.9.2
## [13] fansi_1.0.3 codetools_0.2-18 splines_4.2.2
## [16] cachem_1.0.6 knitr_1.41 polyclip_1.10-4
## [19] jsonlite_1.8.3 ica_1.0-3 cluster_2.1.4
## [22] png_0.1-8 uwot_0.1.14 shiny_1.7.3
## [25] sctransform_0.3.5 spatstat.sparse_3.0-0 compiler_4.2.2
## [28] httr_1.4.4 assertthat_0.2.1 Matrix_1.5-3
## [31] fastmap_1.1.0 lazyeval_0.2.2 limma_3.54.0
## [34] cli_3.4.1 later_1.3.0 htmltools_0.5.3
## [37] tools_4.2.2 igraph_1.3.5 gtable_0.3.1
## [40] glue_1.6.2 RANN_2.6.1 reshape2_1.4.4
## [43] Rcpp_1.0.9 scattermore_0.8 jquerylib_0.1.4
## [46] vctrs_0.5.1 ape_5.6-2 nlme_3.1-160
## [49] spatstat.explore_3.0-5 progressr_0.11.0 lmtest_0.9-40
## [52] spatstat.random_3.0-1 xfun_0.35 stringr_1.4.1
## [55] globals_0.16.2 mime_0.12 miniUI_0.1.1.1
## [58] lifecycle_1.0.3 irlba_2.3.5.1 goftest_1.2-3
## [61] future_1.29.0 MASS_7.3-58.1 zoo_1.8-11
## [64] scales_1.2.1 promises_1.2.0.1 spatstat.utils_3.0-1
## [67] parallel_4.2.2 RColorBrewer_1.1-3 yaml_2.3.6
## [70] reticulate_1.26 pbapply_1.6-0 gridExtra_2.3
## [73] ggrastr_1.0.1 sass_0.4.4 stringi_1.7.8
## [76] highr_0.9 rlang_1.0.6 pkgconfig_2.0.3
## [79] matrixStats_0.63.0 evaluate_0.18 lattice_0.20-45
## [82] tensor_1.5 ROCR_1.0-11 purrr_0.3.5
## [85] labeling_0.4.2 patchwork_1.1.2 htmlwidgets_1.5.4
## [88] cowplot_1.1.1 tidyselect_1.2.0 parallelly_1.32.1
## [91] RcppAnnoy_0.0.20 plyr_1.8.8 magrittr_2.0.3
## [94] R6_2.5.1 generics_0.1.3 DBI_1.1.3
## [97] withr_2.5.0 pillar_1.8.1 fitdistrplus_1.1-8
## [100] survival_3.4-0 abind_1.4-5 sp_1.5-1
## [103] tibble_3.1.8 future.apply_1.10.0 crayon_1.5.2
## [106] KernSmooth_2.23-20 utf8_1.2.2 spatstat.geom_3.0-3
## [109] plotly_4.10.1 rmarkdown_2.18 grid_4.2.2
## [112] data.table_1.14.6 digest_0.6.30 xtable_1.8-4
## [115] tidyr_1.2.1 httpuv_1.6.6 munsell_0.5.0
## [118] beeswarm_0.4.0 viridisLite_0.4.1 vipor_0.4.5
## [121] bslib_0.4.1
