Differential Abundance and Ordination
Load our libraries
# Set up global options for nice reports and keeping figures:
knitr::opts_chunk$set(fig.width=14, fig.height=8, fig.align="center",
warning=FALSE, message=FALSE)
Lets start by loading libraries
library(phyloseq)
library(phangorn)
library(ggplot2)
library(edgeR)
nice_colors = c("#999999", "#E69F00", "#56B4E9","#e98756","#c08160","#5800e6", "#CDDC49", "#C475D3",
"#E94B30", "#233F57", "#FEE659", "#A1CFDD", "#F4755E", "#D6F6F7","#EB6D58", "#6898BF")
Load prior results
load(file=file.path("rdata_objects", "transformed_objects.RData"))
Ordination
#Can view the distance method options with
?distanceMethodList
# can veiw the oridinate methods with
?ordinate
v1.RLE.ord <- ordinate(ps.1logRLE, "MDS", "wunifrac")
p1 = plot_ordination(ps.1logRLE, v1.RLE.ord, type="taxa", color="Phylum", title="taxa")
p1
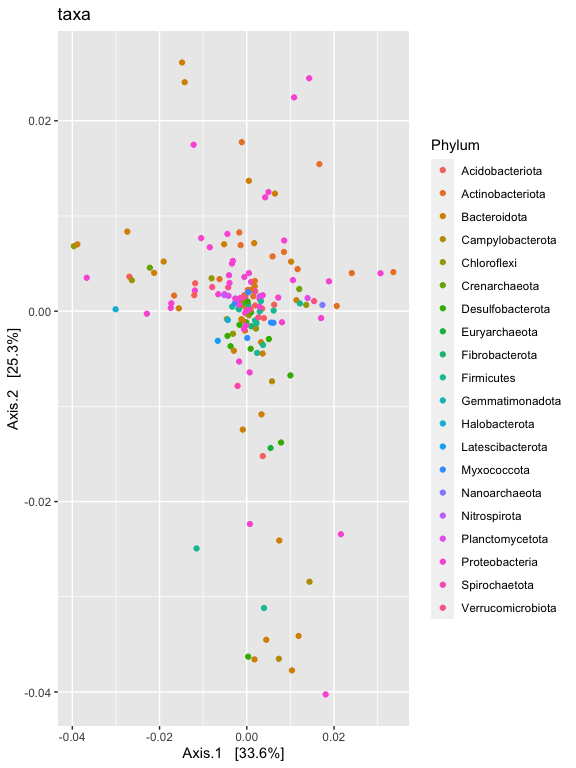
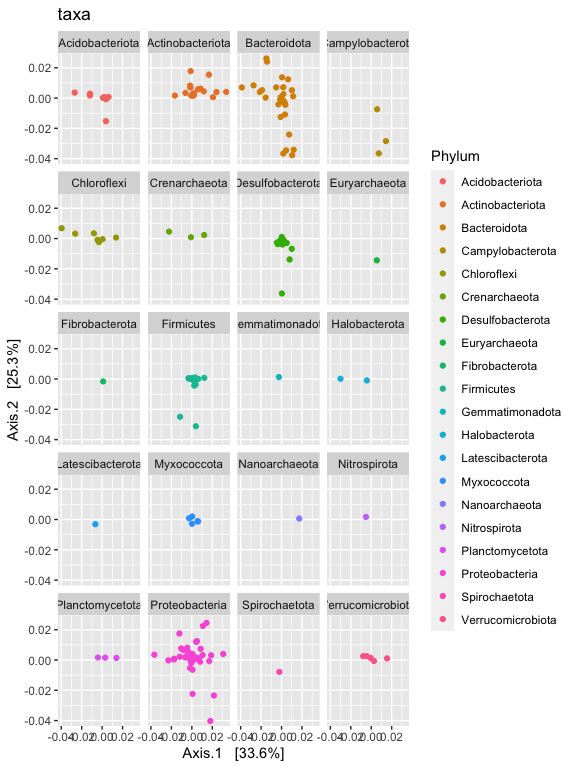
p1 + facet_wrap(~Phylum, 5)
p2 = plot_ordination(ps.1logRLE, v1.RLE.ord, type="samples", color="Temp", shape="Group")
p2

p2 + geom_line() + geom_point(size=5) + ggtitle("samples")
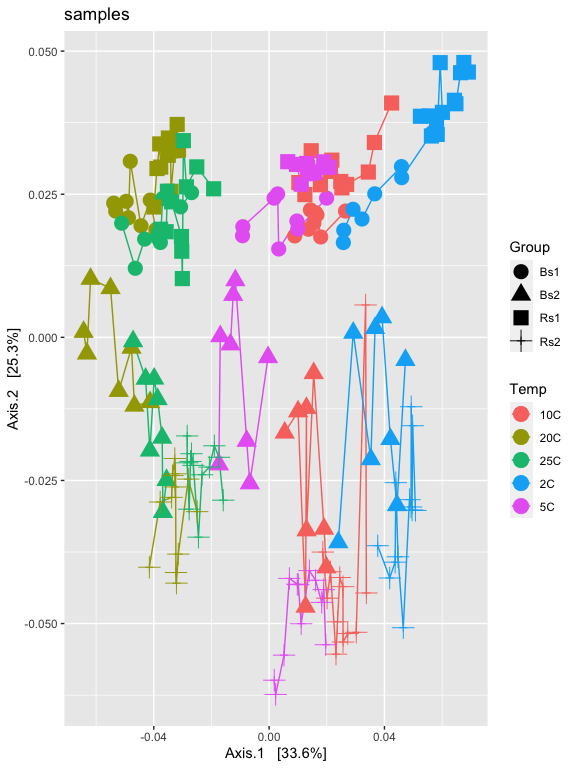
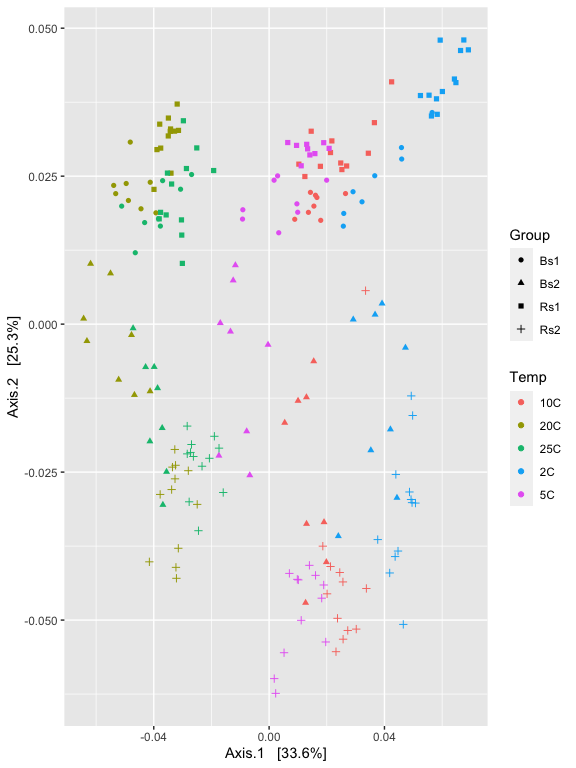
p2
p3 = plot_ordination(ps.1logRLE, v1.RLE.ord, type="biplot", color="Temp", shape="Group") +
scale_shape_manual(values=1:7)
Now try doing oridination with other transformations, such as relative abundance, log. Also looks and see if you can find any trends in the variable Dist_from_edge.
Differential Abundances
For differential abundances we can use RNAseq pipeline EdgeR and limma voom.
m = as(otu_table(ps.1), "matrix")
# Define gene annotations (`genes`) as tax_table
taxonomy = tax_table(ps.1, errorIfNULL=FALSE)
if( !is.null(taxonomy) ){
taxonomy = data.frame(as(taxonomy, "matrix"))
}
# Now turn into a DGEList
d = DGEList(counts=t(m), genes=taxonomy, remove.zeros = TRUE)
## reapply filter
prop = transform_sample_counts(ps.1, function(x) x / sum(x) )
keepTaxa <- ((apply(otu_table(prop) >= 0.005,2,sum,na.rm=TRUE) > 2) | (apply(otu_table(prop) >= 0.05, 2, sum,na.rm=TRUE) > 0))
table(keepTaxa)
## keepTaxa
## FALSE TRUE
## 45 126
d <- d[keepTaxa,]
# Calculate the normalization factors
z = calcNormFactors(d, method="RLE")
# Check for division by zero inside `calcNormFactors`
if( !all(is.finite(z$samples$norm.factors)) ){
stop("Something wrong with edgeR::calcNormFactors on this data,
non-finite $norm.factors, consider changing `method` argument")
}
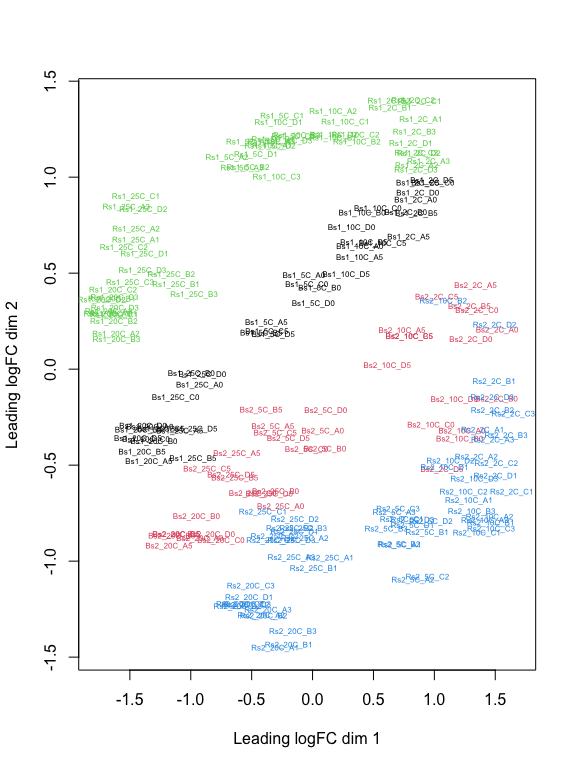
plotMDS(z, col = as.numeric(factor(sample_data(ps.1)$Group)), labels = sample_names(ps.1), cex=0.5)
# Create a model based on Group and Temp
mm <- model.matrix( ~ Group + Temp, data=data.frame(as(sample_data(ps.1),"matrix"))) # specify model with no intercept for easier contrasts
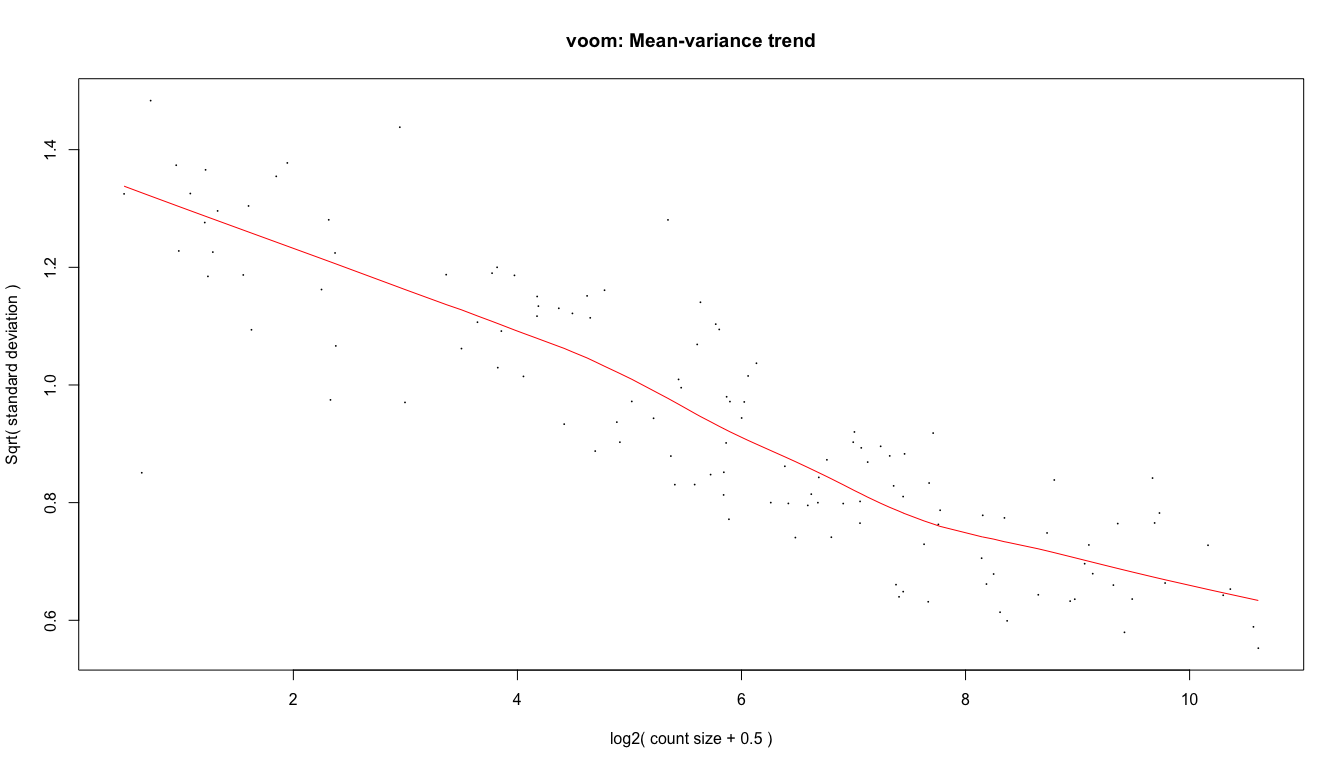
y <- voom(d, mm, plot = T)
fit <- lmFit(y, mm)
head(coef(fit))
## (Intercept) GroupBs2 GroupRs1 GroupRs2 Temp20C Temp25C
## ASV1 15.35742 0.1256581 -0.5698628 0.6392527 -0.8229097 -1.0553334
## ASV3 14.61709 -0.7136901 0.1052358 0.2534002 1.1955619 0.2930345
## ASV6 13.31930 0.6085330 1.3441854 2.0221380 -0.6051854 -0.5160887
## ASV7 16.09468 -1.2648445 0.4935341 -1.6233779 -1.1027966 -0.6994907
## ASV8 13.71681 -0.3855912 2.5926603 1.1961396 -1.0519939 -0.3542017
## ASV9 12.96485 -0.5507289 0.5174640 0.5880462 1.4988344 1.0806996
## Temp2C Temp5C
## ASV1 0.5272901 0.2073291883
## ASV3 -1.2345773 -0.4207734660
## ASV6 0.2173731 -0.4876642016
## ASV7 0.1893725 -0.3187794246
## ASV8 0.4979659 -0.4388517236
## ASV9 -0.4580184 -0.0008135052
# single contrast comparing Temp 5 - 20
contr <- makeContrasts(GroupBs2 = "GroupBs2",
levels = colnames(coef(fit)))
tmp <- contrasts.fit(fit, contr)
tmp <- eBayes(tmp)
tmp2 <- topTable(tmp, coef=1, sort.by = "P", n = Inf)
tmp2$Taxa <- rownames(tmp2)
tmp2 <- tmp2[,c("Taxa","logFC","AveExpr","P.Value","adj.P.Val")]
length(which(tmp2$adj.P.Val < 0.05)) # number of Differentially abundant taxa
## [1] 86
sigtab = cbind(as(tmp2, "data.frame"), as(tax_table(ps.1)[rownames(tmp2), ], "matrix"))
head(sigtab)
## Taxa logFC AveExpr P.Value adj.P.Val Kingdom
## ASV249 ASV249 1.699798 12.19842 2.004571e-42 2.525759e-40 Bacteria
## ASV33 ASV33 -1.501967 14.30675 2.204571e-38 1.388880e-36 Bacteria
## ASV84 ASV84 1.556297 12.96270 2.525979e-33 1.060911e-31 Bacteria
## ASV32 ASV32 1.337056 14.60051 4.617744e-30 1.225911e-28 Bacteria
## ASV7 ASV7 -1.264845 15.11903 4.864726e-30 1.225911e-28 Bacteria
## ASV841 ASV841 2.299733 10.68649 1.326902e-24 2.786494e-23 Bacteria
## Phylum Class Order
## ASV249 Bacteroidota Bacteroidia Bacteroidales
## ASV33 Proteobacteria Alphaproteobacteria Sphingomonadales
## ASV84 Bacteroidota Kryptonia Kryptoniales
## ASV32 Bacteroidota Bacteroidia Bacteroidales
## ASV7 Bacteroidota Bacteroidia Chitinophagales
## ASV841 Spirochaetota Spirochaetia Spirochaetales
## Family Genus Species
## ASV249 SB-5 <NA> <NA>
## ASV33 Sphingomonadaceae <NA> <NA>
## ASV84 BSV26 <NA> <NA>
## ASV32 Bacteroidetes vadinHA17 <NA> <NA>
## ASV7 Chitinophagaceae <NA> <NA>
## ASV841 Spirochaetaceae <NA> <NA>
Cleanup
Save object
dir.create("rdata_objects", showWarnings = FALSE)
save(ps, file=file.path("rdata_objects", "final.Rdata"))
Record session information
sessionInfo()
## R version 4.0.3 (2020-10-10)
## Platform: x86_64-apple-darwin17.0 (64-bit)
## Running under: macOS Big Sur 10.16
##
## Matrix products: default
## BLAS: /Library/Frameworks/R.framework/Versions/4.0/Resources/lib/libRblas.dylib
## LAPACK: /Library/Frameworks/R.framework/Versions/4.0/Resources/lib/libRlapack.dylib
##
## locale:
## [1] en_US.UTF-8/en_US.UTF-8/en_US.UTF-8/C/en_US.UTF-8/en_US.UTF-8
##
## attached base packages:
## [1] stats graphics grDevices utils datasets methods base
##
## other attached packages:
## [1] edgeR_3.32.1 limma_3.46.0 ggplot2_3.3.3 phangorn_2.7.0
## [5] ape_5.5 phyloseq_1.34.0
##
## loaded via a namespace (and not attached):
## [1] Biobase_2.50.0 sass_0.4.0 jsonlite_1.7.2
## [4] splines_4.0.3 foreach_1.5.1 bslib_0.2.5.1
## [7] assertthat_0.2.1 highr_0.9 stats4_4.0.3
## [10] yaml_2.2.1 progress_1.2.2 pillar_1.6.1
## [13] lattice_0.20-44 glue_1.4.2 quadprog_1.5-8
## [16] digest_0.6.27 XVector_0.30.0 colorspace_2.0-1
## [19] htmltools_0.5.1.1 Matrix_1.3-3 plyr_1.8.6
## [22] pkgconfig_2.0.3 zlibbioc_1.36.0 purrr_0.3.4
## [25] scales_1.1.1 tibble_3.1.2 mgcv_1.8-35
## [28] farver_2.1.0 generics_0.1.0 IRanges_2.24.1
## [31] ellipsis_0.3.2 withr_2.4.2 BiocGenerics_0.36.1
## [34] survival_3.2-11 magrittr_2.0.1 crayon_1.4.1
## [37] evaluate_0.14 fansi_0.4.2 nlme_3.1-152
## [40] MASS_7.3-54 vegan_2.5-7 tools_4.0.3
## [43] data.table_1.14.0 prettyunits_1.1.1 hms_1.1.0
## [46] lifecycle_1.0.0 stringr_1.4.0 Rhdf5lib_1.12.1
## [49] S4Vectors_0.28.1 munsell_0.5.0 locfit_1.5-9.4
## [52] cluster_2.1.2 Biostrings_2.58.0 ade4_1.7-16
## [55] compiler_4.0.3 jquerylib_0.1.4 rlang_0.4.11
## [58] rhdf5_2.34.0 grid_4.0.3 iterators_1.0.13
## [61] rhdf5filters_1.2.1 biomformat_1.18.0 igraph_1.2.6
## [64] labeling_0.4.2 rmarkdown_2.8 gtable_0.3.0
## [67] codetools_0.2-18 multtest_2.46.0 DBI_1.1.1
## [70] reshape2_1.4.4 R6_2.5.0 knitr_1.33
## [73] dplyr_1.0.6 utf8_1.2.1 fastmatch_1.1-0
## [76] permute_0.9-5 stringi_1.6.2 parallel_4.0.3
## [79] Rcpp_1.0.6 vctrs_0.3.8 tidyselect_1.1.1
## [82] xfun_0.23
