Linear Models in R
Many bioinformatics applications involving repeatedly fitting linear models to data. Examples include:
- RNA-Seq differential expression analyses
- GWAS (for continuous traits)
- eQTL analyses
- Microarray data analyses
- and on and on ….
Understanding linear modelling in R is critical in implementing these types of analyses.
Scope
- Basics of linear models
- R model syntax
- Understanding contrasts
- Models with continuous covariates
We will not discuss:
- Diagnostic plots
- Data-driven model selection
- Anything that doesn’t scale well when applied to 1000’s of genes/SNPs/proteins
Goals
A full course in linear models would take months, so this is a first introduction rather than a comprehensive how-to. After this session you should:
- Have a general idea of what a linear model is
- Understand where linear models are used in bioinformatics
- Begin to understand model specification in R
Linear models
A linear model is a model for a continuous outcome Y of the form
Y = β0 + β1 X1 + β2 X2 + … + βp Xp + ε
The covariates X can be:
- a continuous variable (age, weight, temperature, etc.)
- Dummy variables coding a categorical covariate (more later)
The β’s are unknown parameters to be estimated, the “coefficients”.
The error term ε is assumed to be normally distributed with a variance that is constant across the range of the data, the “residuals”.
Models with all categorical covariates are referred to as ANOVA models and models with continuous covariates are referred to as linear regression models. These are all linear models, and R doesn’t distinguish between them.
Read in ‘lm_example_data.csv`:
dat <- read.csv("https://raw.githubusercontent.com/ucdavis-bioinformatics-training/2019-Winter-Bioinformatics_Command_Line_and_R_Prerequisites_Workshop/master/Advanced_R/lm_example_data.csv")
dat$treatment <- factor(dat$treatment)
dim(dat)
## [1] 25 6
head(dat)
## sample expression batch treatment time temperature
## 1 1 1.2139625 Batch1 A time1 11.76575
## 2 2 1.4796581 Batch1 A time2 12.16330
## 3 3 1.0878287 Batch1 A time1 10.54195
## 4 4 1.4438585 Batch1 A time2 10.07642
## 5 5 0.6371621 Batch1 A time1 12.03721
## 6 6 2.1226740 Batch1 B time2 13.49573
tail(dat)
## sample expression batch treatment time temperature
## 20 20 4.270954 Batch2 D time2 12.89125
## 21 21 12.197843 Batch2 E time1 20.84079
## 22 22 5.752513 Batch2 E time2 14.83138
## 23 23 9.881167 Batch2 E time1 19.01435
## 24 24 4.655431 Batch2 E time2 16.31208
## 25 25 10.445337 Batch2 E time1 20.29026
str(dat)
## 'data.frame': 25 obs. of 6 variables:
## $ sample : int 1 2 3 4 5 6 7 8 9 10 ...
## $ expression : num 1.214 1.48 1.088 1.444 0.637 ...
## $ batch : chr "Batch1" "Batch1" "Batch1" "Batch1" ...
## $ treatment : Factor w/ 5 levels "A","B","C","D",..: 1 1 1 1 1 2 2 2 2 2 ...
## $ time : chr "time1" "time2" "time1" "time2" ...
## $ temperature: num 11.8 12.2 10.5 10.1 12 ...
Linear models in R
R uses the function lm to fit linear models.
lm minimally requires a model formula.
Formulas (from the help docs)
The ~ operator is basic in the formation of such models. An expression of the form y ~ model is interpreted as a specification that the response y is modelled by a linear predictor specified symbolically by model.
Models consists of a series of terms separated by + operators. The terms themselves consist of variable and factor names separated by : operators. Such a term (:) is interpreted as the interaction of all the variables and factors appearing in the term.
There are a number of other operators that are useful in model formulae, read the help documentation if you need more advanced modeling fomula.
A model with no intercept can be specified as y ~ x + 0 or y ~ 0 + x.
For our data, lets fit a linear model using expression as the outcome and treatment as a categorical covariate:
oneway.model <- lm(expression ~ treatment, data = dat)
In R model syntax, the outcome is on the left side, with covariates (separated by +) following the ~.
oneway.model
##
## Call:
## lm(formula = expression ~ treatment, data = dat)
##
## Coefficients:
## (Intercept) treatmentB treatmentC treatmentD treatmentE
## 1.1725 0.4455 0.9028 2.5537 7.4140
class(oneway.model)
## [1] "lm"
Note that this is a “one-way ANOVA” model.
summary() applied to an lm object will give p-values and other relevant information:
summary(oneway.model)
##
## Call:
## lm(formula = expression ~ treatment, data = dat)
##
## Residuals:
## Min 1Q Median 3Q Max
## -3.9310 -0.5353 0.1790 0.7725 3.6114
##
## Coefficients:
## Estimate Std. Error t value Pr(>|t|)
## (Intercept) 1.1725 0.7783 1.506 0.148
## treatmentB 0.4455 1.1007 0.405 0.690
## treatmentC 0.9028 1.1007 0.820 0.422
## treatmentD 2.5537 1.1007 2.320 0.031 *
## treatmentE 7.4140 1.1007 6.735 1.49e-06 ***
## ---
## Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
##
## Residual standard error: 1.74 on 20 degrees of freedom
## Multiple R-squared: 0.7528, Adjusted R-squared: 0.7033
## F-statistic: 15.22 on 4 and 20 DF, p-value: 7.275e-06
In the output:
- “Coefficients” refer to the β’s
- “Estimate” is the estimate of each coefficient
- “Std. Error” is the standard error of the estimate
- “t value” is the coefficient divided by its standard error
- “Pr(>|t|)” is the p-value for the coefficient
- The residual standard error is the estimate of the variance of ε
- Degrees of freedom is the sample size minus # of coefficients estimated
- R-squared is (roughly) the proportion of variance in the outcome explained by the model
- The F-statistic compares the fit of the model as a whole to the null model (with no covariates)
coef() gives you model coefficients:
coef(oneway.model)
## (Intercept) treatmentB treatmentC treatmentD treatmentE
## 1.1724940 0.4455249 0.9027755 2.5536669 7.4139642
What do the model coefficients mean?
By default, R uses reference group coding or “treatment contrasts”. For categorical covariates, the first factor level (alphabetically by default) is treated as the reference group. The reference group doesn’t get its own coefficient, it is represented by the intercept. Coefficients for other groups are the difference from the reference:
levels(dat$treatment)
## [1] "A" "B" "C" "D" "E"
For our simple design:
(Intercept)is the mean of expression for treatment = AtreatmentBis the mean of expression for treatment = B minus the mean for treatment = AtreatmentCis the mean of expression for treatment = C minus the mean for treatment = A- etc.
# Get means in each treatment
treatmentmeans <- tapply(dat$expression, dat$treatment, mean)
treatmentmeans["A"]
## A
## 1.172494
# Difference in means gives you the "treatmentB" coefficient from oneway.model
treatmentmeans["B"] - treatmentmeans["A"]
## B
## 0.4455249
What if you don’t want reference group coding? Another option is to fit a model without an intercept:
no.intercept.model <- lm(expression ~ 0 + treatment, data = dat) # '0' means 'no intercept' here
summary(no.intercept.model)
##
## Call:
## lm(formula = expression ~ 0 + treatment, data = dat)
##
## Residuals:
## Min 1Q Median 3Q Max
## -3.9310 -0.5353 0.1790 0.7725 3.6114
##
## Coefficients:
## Estimate Std. Error t value Pr(>|t|)
## treatmentA 1.1725 0.7783 1.506 0.147594
## treatmentB 1.6180 0.7783 2.079 0.050717 .
## treatmentC 2.0753 0.7783 2.666 0.014831 *
## treatmentD 3.7262 0.7783 4.787 0.000112 ***
## treatmentE 8.5865 0.7783 11.032 5.92e-10 ***
## ---
## Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
##
## Residual standard error: 1.74 on 20 degrees of freedom
## Multiple R-squared: 0.8878, Adjusted R-squared: 0.8598
## F-statistic: 31.66 on 5 and 20 DF, p-value: 7.605e-09
coef(no.intercept.model)
## treatmentA treatmentB treatmentC treatmentD treatmentE
## 1.172494 1.618019 2.075270 3.726161 8.586458
Without the intercept, the coefficients here estimate the mean in each level of treatment:
treatmentmeans
## A B C D E
## 1.172494 1.618019 2.075270 3.726161 8.586458
The no-intercept model is the SAME model as the reference group coded model, in the sense that it gives the same estimate for any comparison between groups:
Treatment B - treatment A, reference group coded model:
coefs <- coef(oneway.model)
coefs["treatmentB"]
## treatmentB
## 0.4455249
Treatment B - treatment A, no-intercept model:
coefs <- coef(no.intercept.model)
coefs["treatmentB"] - coefs["treatmentA"]
## treatmentB
## 0.4455249
testing contrasts using emmeans
if (!("emmeans" %in% rownames(installed.packages())))
install.packages("emmeans")
library(emmeans)
oneway.model.emm <- emmeans(oneway.model, "treatment")
pairs(oneway.model.emm)
## contrast estimate SE df t.ratio p.value
## A - B -0.446 1.1 20 -0.405 0.9939
## A - C -0.903 1.1 20 -0.820 0.9213
## A - D -2.554 1.1 20 -2.320 0.1797
## A - E -7.414 1.1 20 -6.735 <.0001
## B - C -0.457 1.1 20 -0.415 0.9933
## B - D -2.108 1.1 20 -1.915 0.3416
## B - E -6.968 1.1 20 -6.331 <.0001
## C - D -1.651 1.1 20 -1.500 0.5743
## C - E -6.511 1.1 20 -5.915 0.0001
## D - E -4.860 1.1 20 -4.416 0.0022
##
## P value adjustment: tukey method for comparing a family of 5 estimates
coef(pairs(oneway.model.emm))
## treatment c.1 c.2 c.3 c.4 c.5 c.6 c.7 c.8 c.9 c.10
## A A 1 1 1 1 0 0 0 0 0 0
## B B -1 0 0 0 1 1 1 0 0 0
## C C 0 -1 0 0 -1 0 0 1 1 0
## D D 0 0 -1 0 0 -1 0 -1 0 1
## E E 0 0 0 -1 0 0 -1 0 -1 -1
relevel a variable
You can relevel your treatment, if say treatment “E” should be considered your control and you’d like the intercept to be the control treatment.
dat$treatment <- relevel(dat$treatment, "E")
oneway.model <- lm(expression ~ treatment, data = dat)
coef(oneway.model)
## (Intercept) treatmentA treatmentB treatmentC treatmentD
## 8.586458 -7.413964 -6.968439 -6.511189 -4.860297
summary(oneway.model)
##
## Call:
## lm(formula = expression ~ treatment, data = dat)
##
## Residuals:
## Min 1Q Median 3Q Max
## -3.9310 -0.5353 0.1790 0.7725 3.6114
##
## Coefficients:
## Estimate Std. Error t value Pr(>|t|)
## (Intercept) 8.5865 0.7783 11.032 5.92e-10 ***
## treatmentA -7.4140 1.1007 -6.735 1.49e-06 ***
## treatmentB -6.9684 1.1007 -6.331 3.53e-06 ***
## treatmentC -6.5112 1.1007 -5.915 8.73e-06 ***
## treatmentD -4.8603 1.1007 -4.416 0.000266 ***
## ---
## Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
##
## Residual standard error: 1.74 on 20 degrees of freedom
## Multiple R-squared: 0.7528, Adjusted R-squared: 0.7033
## F-statistic: 15.22 on 4 and 20 DF, p-value: 7.275e-06
# undo releveling so it doesn't mess up remaining examples
dat$treatment <- relevel(dat$treatment, "A")
The Design Matrix
For the RNASeq analysis programs, ex. limma and edgeR, the model is specified through the design matrix.
The design matrix X has one row for each observation and one column for each model coefficient.
Sound complicated? The good news is that the design matrix can be specified through the model.matrix function using the same syntax as for lm, just without a response:
Design matrix for reference group coded model:
X <- model.matrix(~ treatment, data = dat)
X
## (Intercept) treatmentE treatmentB treatmentC treatmentD
## 1 1 0 0 0 0
## 2 1 0 0 0 0
## 3 1 0 0 0 0
## 4 1 0 0 0 0
## 5 1 0 0 0 0
## 6 1 0 1 0 0
## 7 1 0 1 0 0
## 8 1 0 1 0 0
## 9 1 0 1 0 0
## 10 1 0 1 0 0
## 11 1 0 0 1 0
## 12 1 0 0 1 0
## 13 1 0 0 1 0
## 14 1 0 0 1 0
## 15 1 0 0 1 0
## 16 1 0 0 0 1
## 17 1 0 0 0 1
## 18 1 0 0 0 1
## 19 1 0 0 0 1
## 20 1 0 0 0 1
## 21 1 1 0 0 0
## 22 1 1 0 0 0
## 23 1 1 0 0 0
## 24 1 1 0 0 0
## 25 1 1 0 0 0
## attr(,"assign")
## [1] 0 1 1 1 1
## attr(,"contrasts")
## attr(,"contrasts")$treatment
## [1] "contr.treatment"
(Note that “contr.treatment”, or treatment contrasts, is how R refers to reference group coding)
- The first column will always be 1 in every row if your model has an intercept
- The column
treatmentBis 1 if an observation has treatment B and 0 otherwise - The column
treatmentCis 1 if an observation has treatment C and 0 otherwise - etc.
Exercises and Things to Think About
- Use ?lm.fit to see how lm uses the design matrix internally.
- If the response y is log gene expression, the model coefficients are often referred to as log fold-changes. Why does this make sense? (Hint: log(x/y) = log(x) - log(y)).
Adding More Covariates
Batch Adjustment
Suppose we want to adjust for batch differences in our model. We do this by adding the covariate “batch” to the model formula:
batch.model <- lm(expression ~ treatment + batch, data = dat)
summary(batch.model)
##
## Call:
## lm(formula = expression ~ treatment + batch, data = dat)
##
## Residuals:
## Min 1Q Median 3Q Max
## -3.9310 -0.8337 0.0415 0.7725 3.6114
##
## Coefficients:
## Estimate Std. Error t value Pr(>|t|)
## (Intercept) 1.1725 0.7757 1.512 0.147108
## treatmentE 9.1017 1.9263 4.725 0.000147 ***
## treatmentB 0.4455 1.0970 0.406 0.689186
## treatmentC 1.9154 1.4512 1.320 0.202561
## treatmentD 4.2414 1.9263 2.202 0.040231 *
## batchBatch2 -1.6877 1.5834 -1.066 0.299837
## ---
## Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
##
## Residual standard error: 1.735 on 19 degrees of freedom
## Multiple R-squared: 0.7667, Adjusted R-squared: 0.7053
## F-statistic: 12.49 on 5 and 19 DF, p-value: 1.835e-05
For a model with more than one coefficient, summary provides estimates and tests for each coefficient adjusted for all the other coefficients in the model.
Recall:
Y = β0 + β1 X1 + β2 X2 + … + βp Xp + ε
model.matrix help you visualize the covariates for each sample.
model.matrix(~treatment + batch, data = dat)
## (Intercept) treatmentE treatmentB treatmentC treatmentD batchBatch2
## 1 1 0 0 0 0 0
## 2 1 0 0 0 0 0
## 3 1 0 0 0 0 0
## 4 1 0 0 0 0 0
## 5 1 0 0 0 0 0
## 6 1 0 1 0 0 0
## 7 1 0 1 0 0 0
## 8 1 0 1 0 0 0
## 9 1 0 1 0 0 0
## 10 1 0 1 0 0 0
## 11 1 0 0 1 0 0
## 12 1 0 0 1 0 0
## 13 1 0 0 1 0 1
## 14 1 0 0 1 0 1
## 15 1 0 0 1 0 1
## 16 1 0 0 0 1 1
## 17 1 0 0 0 1 1
## 18 1 0 0 0 1 1
## 19 1 0 0 0 1 1
## 20 1 0 0 0 1 1
## 21 1 1 0 0 0 1
## 22 1 1 0 0 0 1
## 23 1 1 0 0 0 1
## 24 1 1 0 0 0 1
## 25 1 1 0 0 0 1
## attr(,"assign")
## [1] 0 1 1 1 1 2
## attr(,"contrasts")
## attr(,"contrasts")$treatment
## [1] "contr.treatment"
##
## attr(,"contrasts")$batch
## [1] "contr.treatment"
and coefficients of the model are
coef(batch.model)
## (Intercept) treatmentE treatmentB treatmentC treatmentD batchBatch2
## 1.1724940 9.1016661 0.4455249 1.9153967 4.2413688 -1.6877019
The response if then of course
dat$expression
## [1] 1.2139625 1.4796581 1.0878287 1.4438585 0.6371621 2.1226740
## [7] 1.1361548 2.4896243 1.6358831 0.7057580 2.2542310 3.9215504
## [13] 3.7500544 0.4914460 -0.0409341 4.0002658 3.6059810 2.2549723
## [19] 4.4986315 4.2709538 12.1978430 5.7525135 9.8811666 4.6554312
## [25] 10.4453366
Two-Way ANOVA Models
Suppose our experiment involves two factors, treatment and time. lm can be used to fit a two-way ANOVA model:
twoway.model <- lm(expression ~ treatment*time, data = dat)
summary(twoway.model)
##
## Call:
## lm(formula = expression ~ treatment * time, data = dat)
##
## Residuals:
## Min 1Q Median 3Q Max
## -2.0287 -0.4463 0.1082 0.4915 1.7623
##
## Coefficients:
## Estimate Std. Error t value Pr(>|t|)
## (Intercept) 0.97965 0.69239 1.415 0.17752
## treatmentE 9.86180 0.97918 10.071 4.55e-08 ***
## treatmentB 0.40637 1.09476 0.371 0.71568
## treatmentC 1.00813 0.97918 1.030 0.31953
## treatmentD 3.07266 1.09476 2.807 0.01328 *
## timetime2 0.48211 1.09476 0.440 0.66594
## treatmentE:timetime2 -6.11958 1.54822 -3.953 0.00128 **
## treatmentB:timetime2 -0.09544 1.54822 -0.062 0.95166
## treatmentC:timetime2 -0.26339 1.54822 -0.170 0.86718
## treatmentD:timetime2 -1.02568 1.54822 -0.662 0.51771
## ---
## Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
##
## Residual standard error: 1.199 on 15 degrees of freedom
## Multiple R-squared: 0.912, Adjusted R-squared: 0.8591
## F-statistic: 17.26 on 9 and 15 DF, p-value: 2.242e-06
coef(twoway.model)
## (Intercept) treatmentE treatmentB
## 0.97965110 9.86179766 0.40636785
## treatmentC treatmentD timetime2
## 1.00813264 3.07265513 0.48210723
## treatmentE:timetime2 treatmentB:timetime2 treatmentC:timetime2
## -6.11958364 -0.09544075 -0.26339279
## treatmentD:timetime2
## -1.02568281
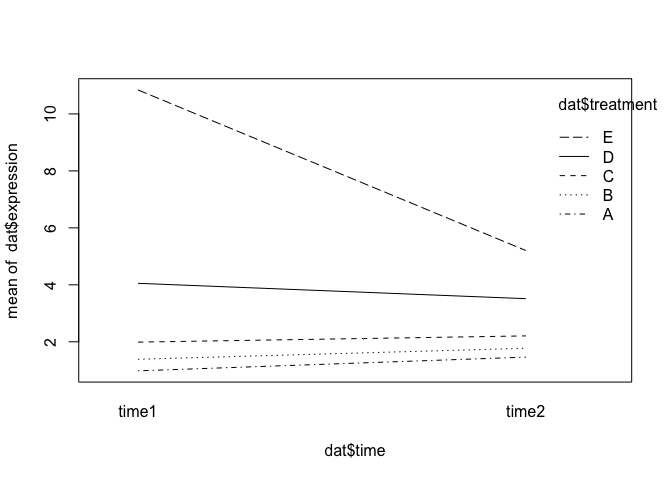
The notation treatment*time refers to treatment, time, and the interaction effect of treatment by time. (This is different from other statistical software).
Interpretation of coefficients:
- Each coefficient for treatment represents the difference between the indicated group and the reference group at the reference level for the other covariates
- For example, “treatmentB” is the difference in expression between treatment B and treatment A at time 1
- Similarly, “timetime2” is the difference in expression between time2 and time1 for treatment A
- The interaction effects (coefficients with “:”) estimate the difference between treatment groups in the effect of time
- The interaction effects ALSO estimate the difference between times in the effect of treatment
To estimate the difference between treatment B and treatment A at time 2, we need to include the interaction effects:
# A - B at time 2
coefs <- coef(twoway.model)
coefs["treatmentB"] + coefs["treatmentB:timetime2"]
## treatmentB
## 0.3109271
We can see from summary that one of the interaction effects is significant. Here’s what that interaction effect looks like graphically:
interaction.plot(x.factor = dat$time, trace.factor = dat$treatment, response = dat$expression)
Another Parameterization
In a multifactor model, estimating contrasts can be fiddly, especially with lots of factors or levels. Here is an equivalent way to estimate the same two-way ANOVA model that gives easier contrasts:
First, define a new variable that combines the information from the treatment and time variables
dat$tx.time <- interaction(dat$treatment, dat$time)
dat$tx.time
## [1] A.time1 A.time2 A.time1 A.time2 A.time1 B.time2 B.time1 B.time2 B.time1
## [10] B.time2 C.time1 C.time2 C.time1 C.time2 C.time1 D.time2 D.time1 D.time2
## [19] D.time1 D.time2 E.time1 E.time2 E.time1 E.time2 E.time1
## 10 Levels: A.time1 E.time1 B.time1 C.time1 D.time1 A.time2 E.time2 ... D.time2
table(dat$tx.time, dat$treatment)
##
## A E B C D
## A.time1 3 0 0 0 0
## E.time1 0 3 0 0 0
## B.time1 0 0 2 0 0
## C.time1 0 0 0 3 0
## D.time1 0 0 0 0 2
## A.time2 2 0 0 0 0
## E.time2 0 2 0 0 0
## B.time2 0 0 3 0 0
## C.time2 0 0 0 2 0
## D.time2 0 0 0 0 3
table(dat$tx.time, dat$time)
##
## time1 time2
## A.time1 3 0
## E.time1 3 0
## B.time1 2 0
## C.time1 3 0
## D.time1 2 0
## A.time2 0 2
## E.time2 0 2
## B.time2 0 3
## C.time2 0 2
## D.time2 0 3
Next, fit a one-way ANOVA model with the new covariate. Don’t include an intercept in the model.
other.2way.model <- lm(expression ~ 0 + tx.time, data = dat)
summary(other.2way.model)
##
## Call:
## lm(formula = expression ~ 0 + tx.time, data = dat)
##
## Residuals:
## Min 1Q Median 3Q Max
## -2.0287 -0.4463 0.1082 0.4915 1.7623
##
## Coefficients:
## Estimate Std. Error t value Pr(>|t|)
## tx.timeA.time1 0.9797 0.6924 1.415 0.177524
## tx.timeE.time1 10.8414 0.6924 15.658 1.06e-10 ***
## tx.timeB.time1 1.3860 0.8480 1.634 0.122968
## tx.timeC.time1 1.9878 0.6924 2.871 0.011662 *
## tx.timeD.time1 4.0523 0.8480 4.779 0.000244 ***
## tx.timeA.time2 1.4618 0.8480 1.724 0.105290
## tx.timeE.time2 5.2040 0.8480 6.137 1.90e-05 ***
## tx.timeB.time2 1.7727 0.6924 2.560 0.021751 *
## tx.timeC.time2 2.2065 0.8480 2.602 0.020018 *
## tx.timeD.time2 3.5087 0.6924 5.068 0.000139 ***
## ---
## Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
##
## Residual standard error: 1.199 on 15 degrees of freedom
## Multiple R-squared: 0.9601, Adjusted R-squared: 0.9334
## F-statistic: 36.06 on 10 and 15 DF, p-value: 1.14e-08
coef(other.2way.model)
## tx.timeA.time1 tx.timeE.time1 tx.timeB.time1 tx.timeC.time1 tx.timeD.time1
## 0.9796511 10.8414488 1.3860189 1.9877837 4.0523062
## tx.timeA.time2 tx.timeE.time2 tx.timeB.time2 tx.timeC.time2 tx.timeD.time2
## 1.4617583 5.2039723 1.7726854 2.2064982 3.5087306
We get the same estimates for the effect of treatment B vs. A at time 1:
c1 <- coef(twoway.model)
c1["treatmentB"]
## treatmentB
## 0.4063679
c2 <- coef(other.2way.model)
c2["tx.timeB.time1"] - c2["tx.timeA.time1"]
## tx.timeB.time1
## 0.4063679
We get the same estimates for the effect of treatment B vs. A at time 2:
c1 <- coef(twoway.model)
c1["treatmentB"] + c1["treatmentB:timetime2"]
## treatmentB
## 0.3109271
c2 <- coef(other.2way.model)
c2["tx.timeB.time2"] - c2["tx.timeA.time2"]
## tx.timeB.time2
## 0.3109271
And we get the same estimates for the interaction effect (remembering that an interaction effect here is a difference of differences):
c1 <- coef(twoway.model)
c1["treatmentB:timetime2"]
## treatmentB:timetime2
## -0.09544075
c2 <- coef(other.2way.model)
(c2["tx.timeB.time2"] - c2["tx.timeA.time2"]) - (c2["tx.timeB.time1"] - c2["tx.timeA.time1"])
## tx.timeB.time2
## -0.09544075
(See https://www.bioconductor.org/packages/3.7/bioc/vignettes/limma/inst/doc/usersguide.pdf for more details on this parameterization)
Exercises and Things to Think About
- How much do the parameter estimates for treatment change when batch is added?
- The data frame dat has a column called ‘temperature’. What formula would you use if you wanted to look at differences between treatments, adjusting for temperature?
Continuous Covariates
Linear models with continuous covariates (“regression models”) are fitted in much the same way:
continuous.model <- lm(expression ~ temperature, data = dat)
summary(continuous.model)
##
## Call:
## lm(formula = expression ~ temperature, data = dat)
##
## Residuals:
## Min 1Q Median 3Q Max
## -1.87373 -0.67875 -0.07922 1.00672 1.89564
##
## Coefficients:
## Estimate Std. Error t value Pr(>|t|)
## (Intercept) -9.40718 0.93724 -10.04 7.13e-10 ***
## temperature 0.97697 0.06947 14.06 8.77e-13 ***
## ---
## Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
##
## Residual standard error: 1.054 on 23 degrees of freedom
## Multiple R-squared: 0.8958, Adjusted R-squared: 0.8913
## F-statistic: 197.8 on 1 and 23 DF, p-value: 8.768e-13
coef(continuous.model)
## (Intercept) temperature
## -9.4071796 0.9769656
For the above model, the intercept is the expression at temperature 0 and the “temperature” coefficient is the slope, or how much expression increases for each unit increase in temperature:
coefs <- coef(continuous.model)
plot(expression ~ temperature, data = dat)
abline(coefs)
text(x = 12, y = 10, paste0("expression = ", round(coefs[1], 2), "+", round(coefs[2], 2), "*temperature"))
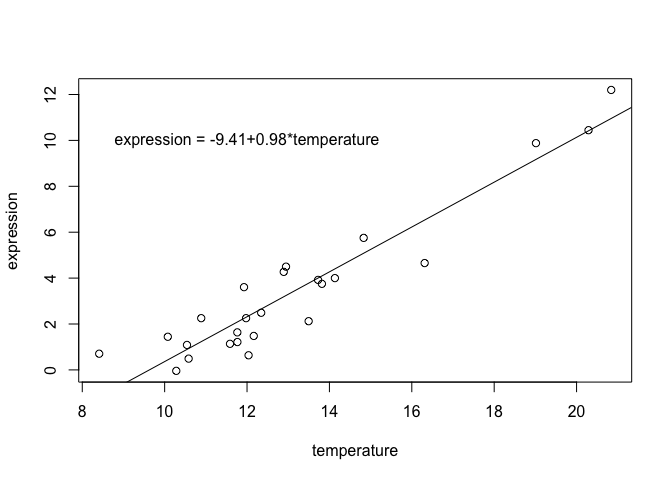
The slope from a linear regression model is related to but not identical to the Pearson correlation coefficient:
cor.test(dat$expression, dat$temperature)
##
## Pearson's product-moment correlation
##
## data: dat$expression and dat$temperature
## t = 14.063, df = 23, p-value = 8.768e-13
## alternative hypothesis: true correlation is not equal to 0
## 95 percent confidence interval:
## 0.8807176 0.9764371
## sample estimates:
## cor
## 0.9464761
summary(continuous.model)
##
## Call:
## lm(formula = expression ~ temperature, data = dat)
##
## Residuals:
## Min 1Q Median 3Q Max
## -1.87373 -0.67875 -0.07922 1.00672 1.89564
##
## Coefficients:
## Estimate Std. Error t value Pr(>|t|)
## (Intercept) -9.40718 0.93724 -10.04 7.13e-10 ***
## temperature 0.97697 0.06947 14.06 8.77e-13 ***
## ---
## Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
##
## Residual standard error: 1.054 on 23 degrees of freedom
## Multiple R-squared: 0.8958, Adjusted R-squared: 0.8913
## F-statistic: 197.8 on 1 and 23 DF, p-value: 8.768e-13
Notice that the p-values for the correlation and the regression slope are identical.
Scaling and centering both variables yields a regression slope equal to the correlation coefficient:
scaled.mod <- lm(scale(expression) ~ scale(temperature), data = dat)
coef(scaled.mod)[2]
## scale(temperature)
## 0.9464761
cor(dat$expression, dat$temperature)
## [1] 0.9464761
Exercises and things to think about
- Look at the documentation for formula again using ?formula. How would you change the formula statement if you wanted to add a quadratic term? Is a model with a quadratic term still a linear model?
- Convert temperature to Farenheit by replacing temperature with I(9/5*temperature + 32) in the model formula. Does the p-value for the association with expression change?
- Look at the documentation for limma here to see a bioinformatics application of what you just learned.
- For your experiment, what would the model formula look like?
